Question: no + (1 - 0) ln(1 - 0) + A%(1 - 0))+ 2. The free energy of mixing per unit volume can be expressed as:
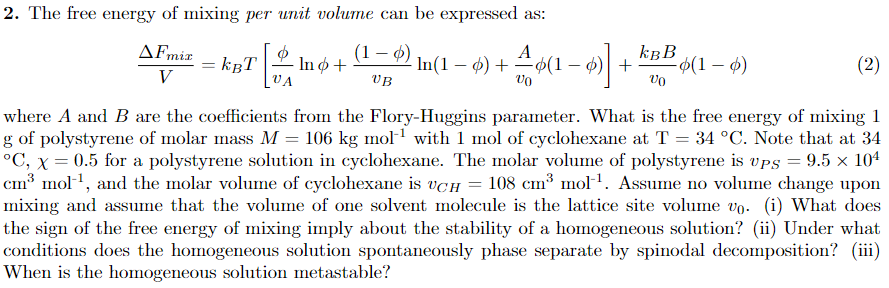
no + (1 - 0) ln(1 - 0) + A%(1 - 0))+ 2. The free energy of mixing per unit volume can be expressed as: AFmir 1-0 kBT kBB In - 1 -0(1-0) (2) V UA VB VO VO where A and B are the coefficients from the Flory-Huggins parameter. What is the free energy of mixing 1 g of polystyrene of molar mass M = 106 kg moll with 1 mol of cyclohexane at T = 34 C. Note that at 34 C, x = 0.5 for a polystyrene solution in cyclohexane. The molar volume of polystyrene is ups = 9.5 x 104 cm mol?, and the molar volume of cyclohexane is vch = 108 cm mol!. Assume no volume change upon mixing and assume that the volume of one solvent molecule is the lattice site volume vo. (i) What does the sign of the free energy of mixing imply about the stability of a homogeneous solution? (i) Under what conditions does the homogeneous solution spontaneously phase separate by spinodal decomposition? (iii) When is the homogeneous solution metastable
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


