Question: take 2 ml from 3M HCl solution and complete it to 60 ml by adding water. Then, add 0.5 ml methyl orange and titrate it
take 2 ml from 3M HCl solution and complete it to 60 ml by adding water. Then, add 0.5 ml methyl orange and titrate it with 1M NaOH solution. the volume of the base used is 6ml. To confirm the molarity of acid you used, recalculate its molartiy according to used amount of base. Show calculations.
need STEP by Step answer with all the Calculations in this form.
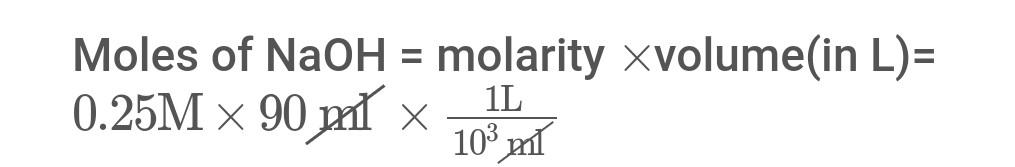
Moles of NaOH= molarity volume ( in L)= 0.25M90prl103mx1L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


