Question: No graph is needed. you are only given the table The irreversible gas-phase dimeriztion A1/2A2 is carried out at 8.2atm in a stirred contained-solids reactor
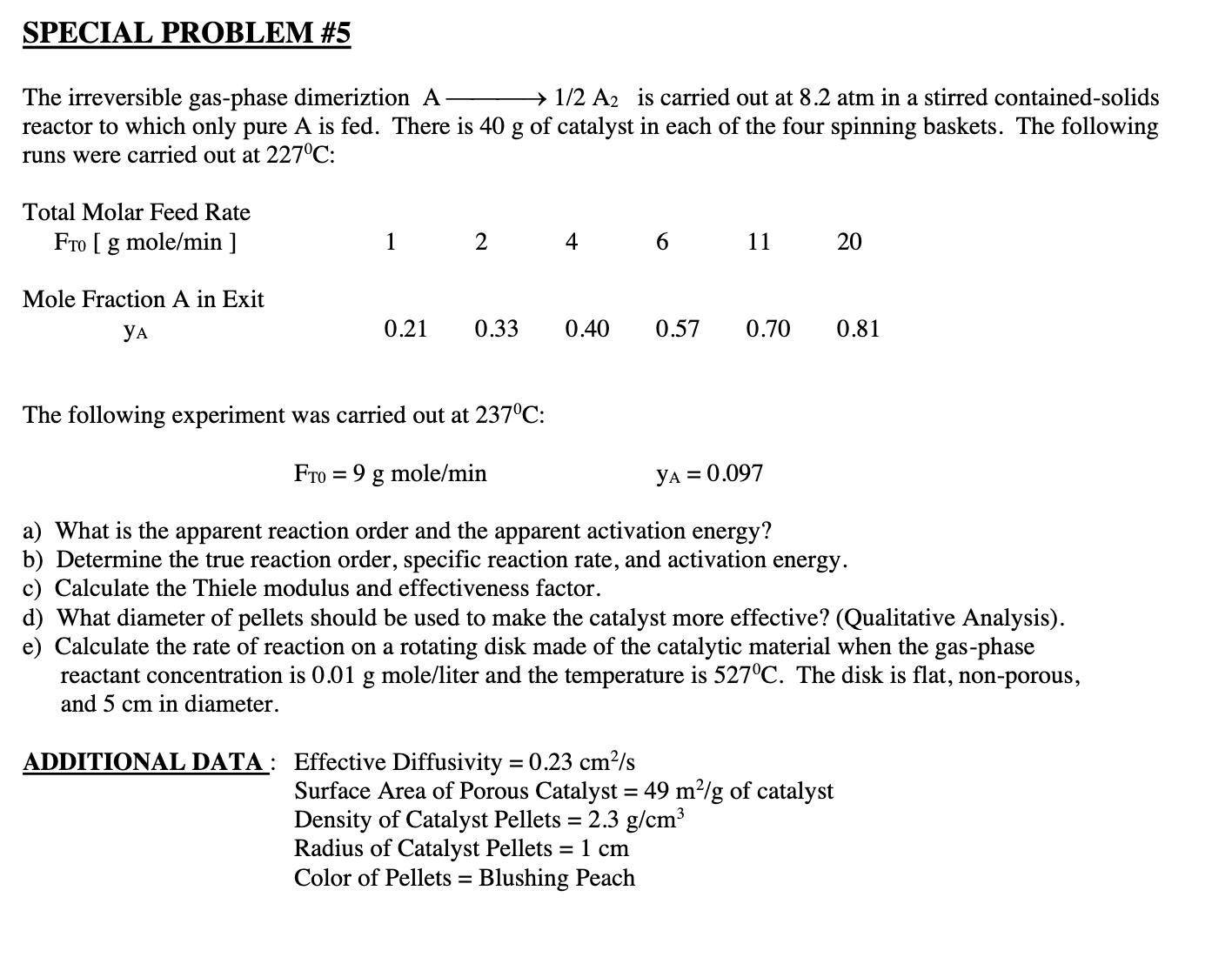
The irreversible gas-phase dimeriztion A1/2A2 is carried out at 8.2atm in a stirred contained-solids reactor to which only pure A is fed. There is 40g of catalyst in each of the four spinning baskets. The following runs were carried out at 227C : The following experiment was carried out at 237C : FT0=9gmole/minyA=0.097 a) What is the apparent reaction order and the apparent activation energy? b) Determine the true reaction order, specific reaction rate, and activation energy. c) Calculate the Thiele modulus and effectiveness factor. d) What diameter of pellets should be used to make the catalyst more effective? (Qualitative Analysis). e) Calculate the rate of reaction on a rotating disk made of the catalytic material when the gas-phase reactant concentration is 0.01g mole/liter and the temperature is 527C. The disk is flat, non-porous, and 5cm in diameter. ADDITIONAL DATA: Effective Diffusivity =0.23cm2/s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


