Question: no work needed just answer for both plzzz Consider the reaction: H2(g)+C2H4(g)C2H6(g) Using standard thermodynamic data at 298K, calculate the free energy change when 1.71
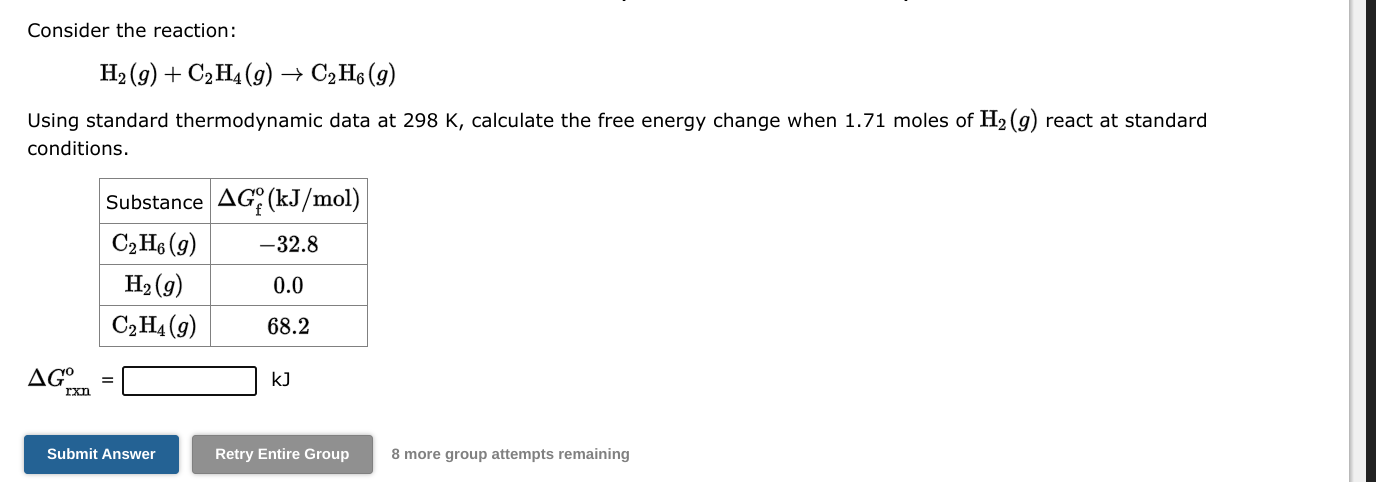
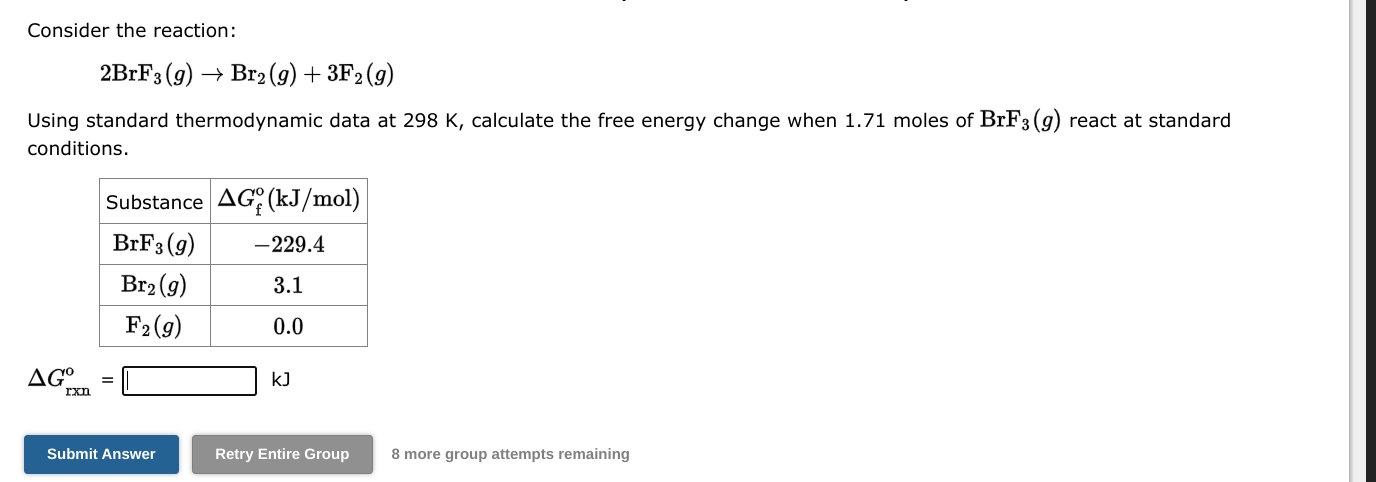 no work needed just answer for both plzzz
no work needed just answer for both plzzz
Consider the reaction: H2(g)+C2H4(g)C2H6(g) Using standard thermodynamic data at 298K, calculate the free energy change when 1.71 moles of H2(g) react at standard conditions. Grxno=kJ 8 more group attempts remaining Consider the reaction: 2BrF3(g)Br2(g)+3F2(g) Using standard thermodynamic data at 298K, calculate the free energy change when 1.71 moles of BrF3(g) react at standard conditions. Grxno=kJ 8 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


