Question: Nomenclature - lonic Compounds containing Polyatomic compounds Nomenclature - Ionic Compounds containing Polyatomic compounds Give the correct formulas for these compounds copper(II) nitrate' iron(II)
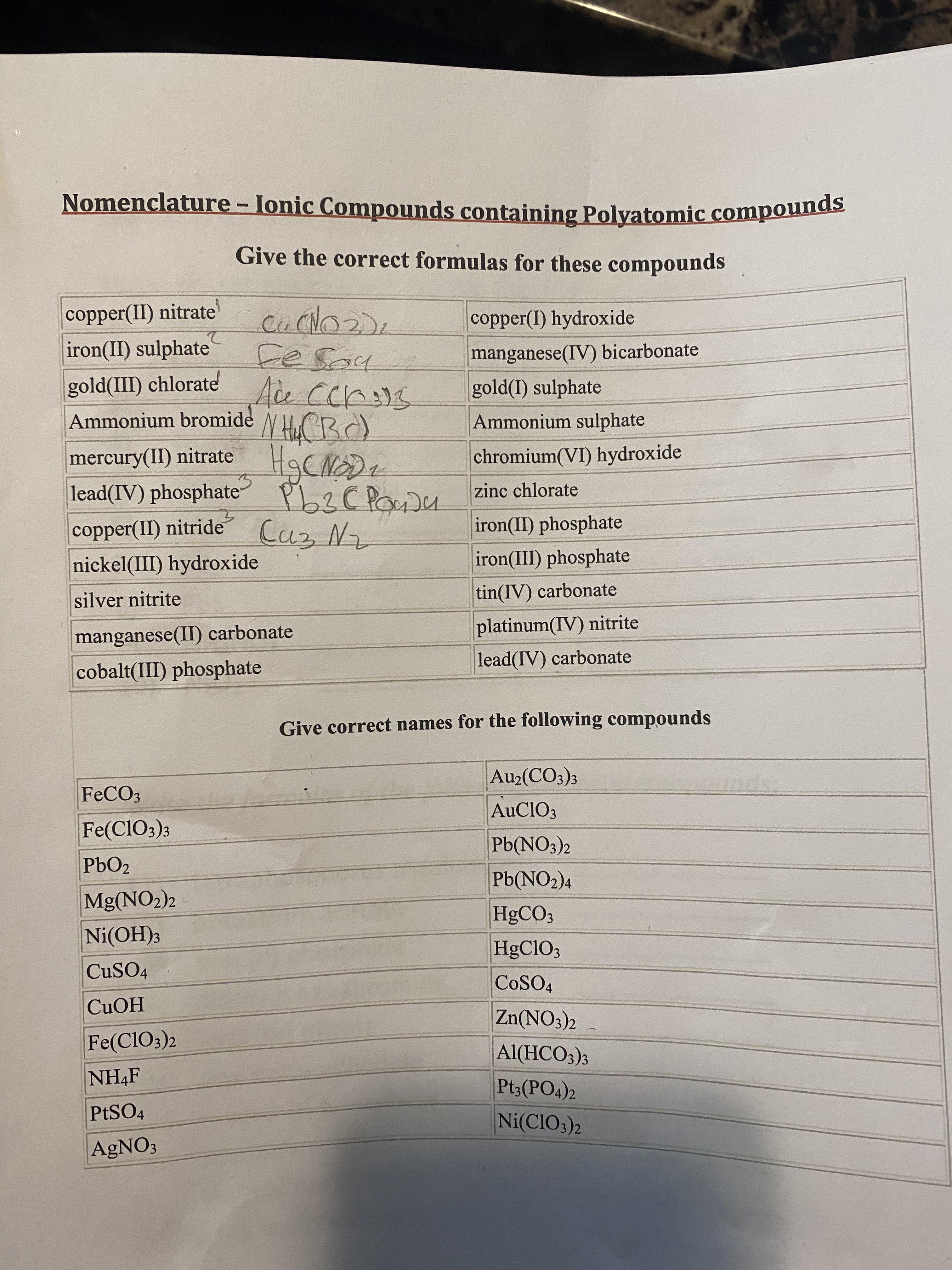
Nomenclature - lonic Compounds containing Polyatomic compounds Nomenclature - Ionic Compounds containing Polyatomic compounds Give the correct formulas for these compounds copper(II) nitrate' iron(II) sulphate gold(III) chlorate Ace Ccr)S copper(I) hydroxide Fesoy manganese(IV) bicarbonate gold(I) sulphate Ammonium bromide Ammonium sulphate mercury(II) nitrate chromium(VI) hydroxide lead(IV) phosphate PLZC PocDu zinc chlorate copper(II) nitride Caz Nz iron(II) phosphate nickel(III) hydroxide iron(III) phosphate silver nitrite tin(IV) carbonate platinum(IV) nitrite lead(IV) carbonate manganese(II) carbonate cobalt(III) phosphate Give correct names for the following compounds Au2(CO3)3 FECO3 nd AuCIO3 Fe(CIO3)3 Pb(NO3)2 PbO2 Pb(NO2)4 Mg(NO2)2 H9CO3 Ni(OH)3 HgClO3 CUSO4 COSO4 CUOH Zn(NO3)2 Fe(Cl03)2 Al(HCO3)3 Pt3(PO4)2 NH4F PESO4 Ni(CIO3)2 AGNO3
Step by Step Solution
3.52 Rating (159 Votes )
There are 3 Steps involved in it
Solution Ionic compounds are made up of positively charged ions known as cations and negatively charged ions known as anions The positive and negative charges are balanced in an ionic compound such th... View full answer

Get step-by-step solutions from verified subject matter experts


