Question: Not sure if this answer is correct. k = 27 any help would be greatly appreciated! Use the rate law you have formulated in Question
Not sure if this answer is correct. k = 27
any help would be greatly appreciated!
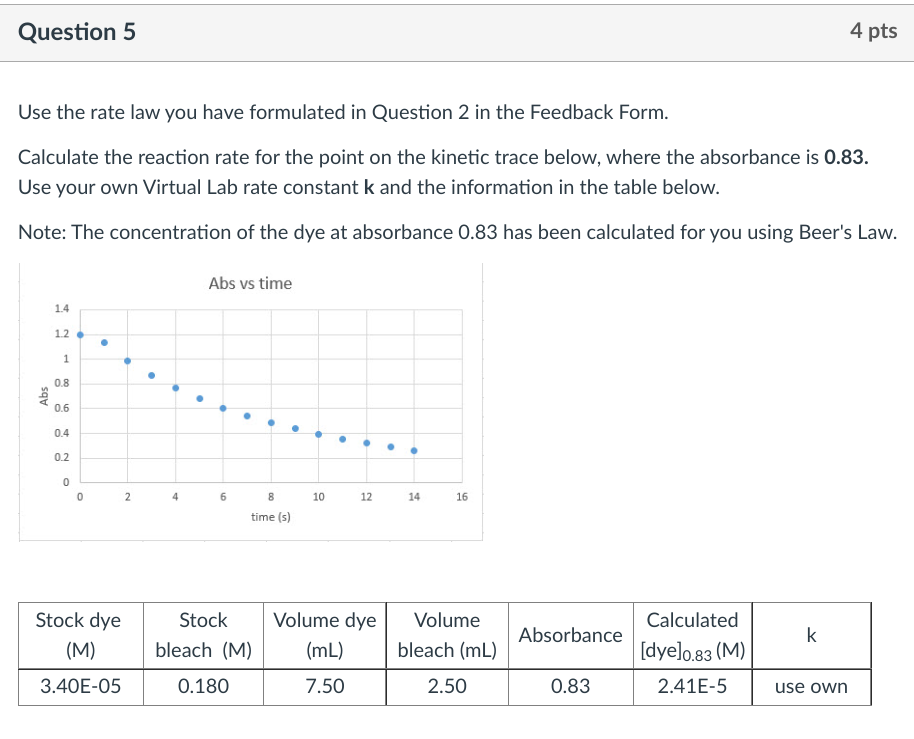
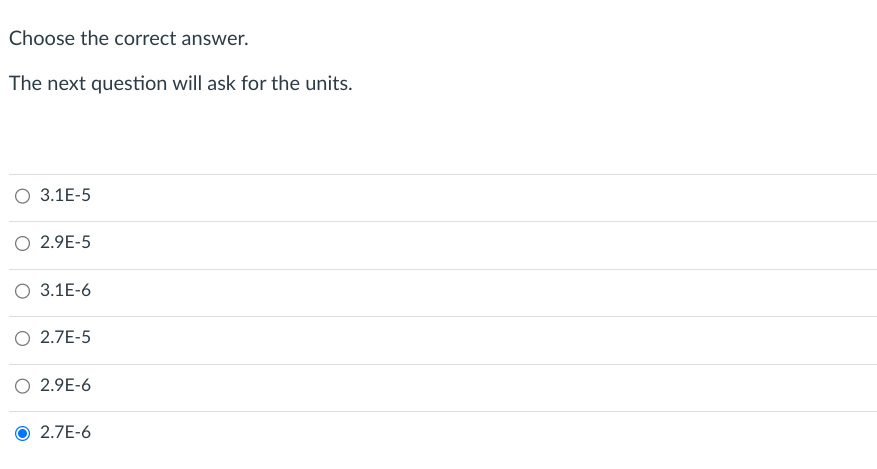
Use the rate law you have formulated in Question 2 in the Feedback Form. Calculate the reaction rate for the point on the kinetic trace below, where the absorbance is 0.83. Use your own Virtual Lab rate constant k and the information in the table below. Note: The concentration of the dye at absorbance 0.83 has been calculated for you using Beer's Law. Choose the correct answer. The next question will ask for the units. \begin{tabular}{c} 3.1E5 \\ \hline 2.9E5 \\ \hline 3.1E6 \\ \hline 2.7E5 \\ \hline 2.9E6 \\ \hline 2.7E6 \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


