Question: For each of the reactions below write the elementary rate law with respect to reactant A. (Use kA as the forward reaction rate constant and
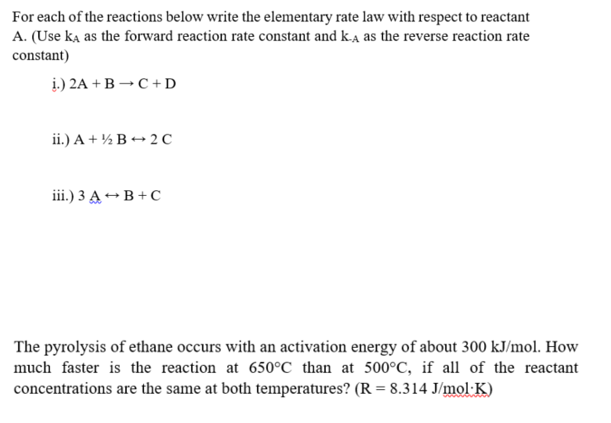
For each of the reactions below write the elementary rate law with respect to reactant A. (Use kA as the forward reaction rate constant and kA as the reverse reaction rate constant) i.) 2A+BC+D ii.) A+1/2B2C iii.) 3AB+C The pyrolysis of ethane occurs with an activation energy of about 300kJ/mol. How much faster is the reaction at 650C than at 500C, if all of the reactant concentrations are the same at both temperatures? (R=8.314J/molK)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


