Question: note that X=32 L/min Question 3 Data in Table Q3 shows the gas-phase reaction of A and B at 200 C. A+B - 20 This
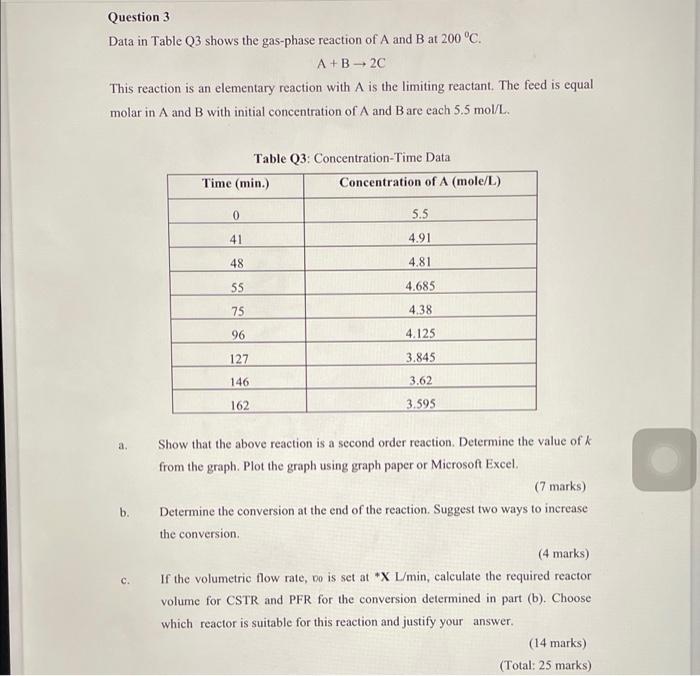
Question 3 Data in Table Q3 shows the gas-phase reaction of A and B at 200 C. A+B - 20 This reaction is an elementary reaction with A is the limiting reactant. The feed is equal molar in A and B with initial concentration of A and B are cach 5.5 mol/L. Table Q3: Concentration-Time Data Time (min.) Concentration of A (mole/L) 0 5.5 41 4.91 48 4.81 55 4.685 75 4.38 96 4.125 3.845 127 146 3.62 162 3.595 b. Show that the above reaction is a second order reaction. Determine the value of k from the graph. Plot the graph using graph paper or Microsoft Excel (7 marks) Determine the conversion at the end of the reaction. Suggest two ways to increase the conversion (4 marks) If the volumetric flow rate, co is set at *X L/min, calculate the required reactor volume for CSTR and PFR for the conversion determined in part (b). Choose which reactor is suitable for this reaction and justify your answer. (14 marks) (Total: 25 marks) c
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


