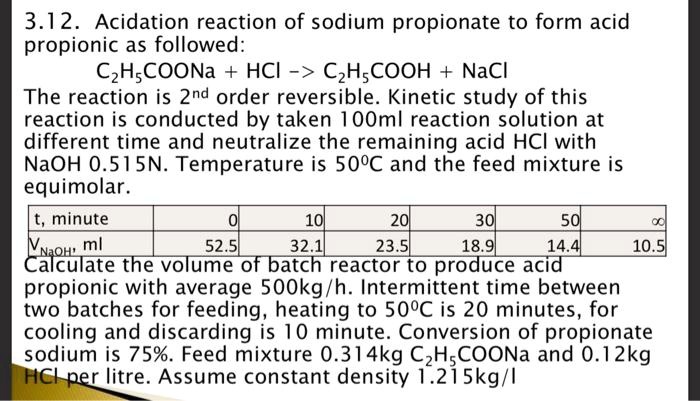
Question: note. The reaction is direct and reverse 2nd order. 3.12. Acidation reaction of sodium propionate to form acid propionic as followed: C2H5COONa+HCl>C2H5COOH+NaCl The reaction is
note. The reaction is direct and reverse 2nd order.
3.12. Acidation reaction of sodium propionate to form acid propionic as followed: C2H5COONa+HCl>C2H5COOH+NaCl The reaction is 2nd order reversible. Kinetic study of this reaction is conducted by taken 100ml reaction solution at different time and neutralize the remaining acid HCl with NaOH0.515N. Temperature is 50C and the feed mixture is equimolar. Calculate the volume of batch reactor to produce acid propionic with average 500kg/h. Intermittent time between two batches for feeding, heating to 50C is 20 minutes, for cooling and discarding is 10 minute. Conversion of propionate sodium is 75%. Feed mixture 0.314kgC2H5COONa and 0.12kg per litre. Assume constant density 1.215kg/I
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


