Question: Note* There is an answer in here. The hint given to me is have a proper basis PROBLEM 3 (25%) In preparing 5.00 moles of
Note* There is an answer in here. The hint given to me is have a proper basis
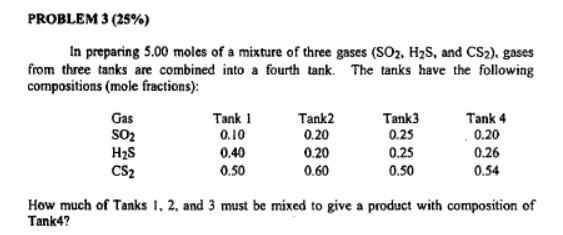
PROBLEM 3 (25%) In preparing 5.00 moles of a mixture of three gases (SO2, H2S, and CS2), gases from three tanks are combined into a fourth tank. The tanks have the following compositions (mole fractions): Gas Tank 1 Tank2 Tank3 Tank 4 SO2 0.10 0.25 0.20 H2S 0.40 0.20 0.25 0.26 CS2 0.50 0.60 0.50 0.54 How much of Tanks 1.2 and 3 must be mixed to give a product with composition of Tank4? 0.20
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


