Question: Note: You will be required to use data from the NIST Webbook ( found here ) for appropriate properties to solve the problem. A tutorial
Note: You will be required to use data from the NIST Webbook found here for appropriate properties to
solve the problem. A "tutorial video" for using the Webbook has also been uploaded in this week's content.
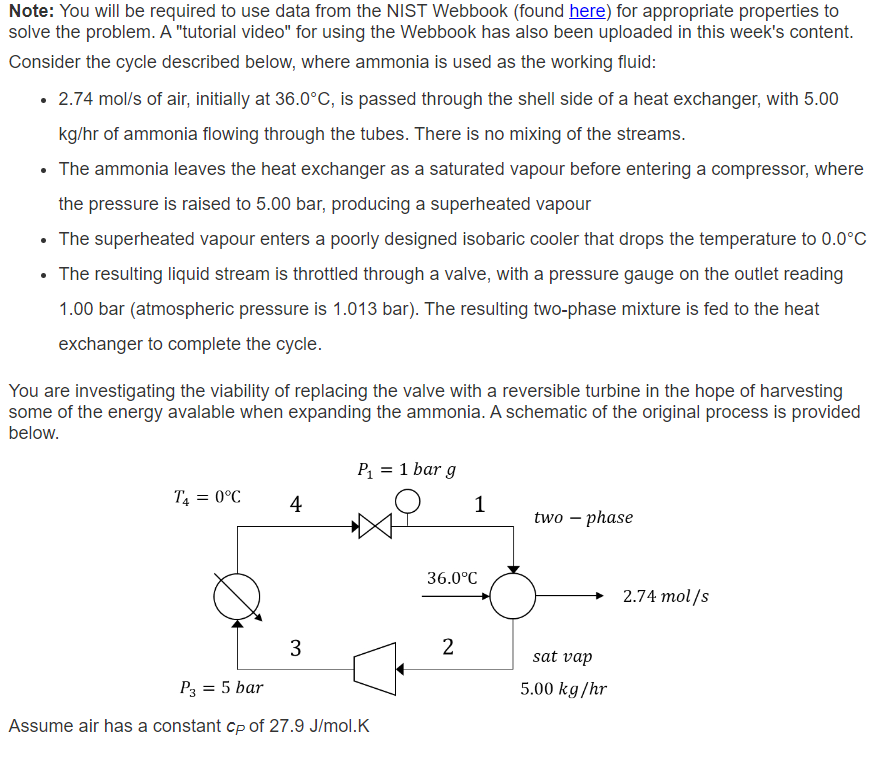
Consider the cycle described below, where ammonia is used as the working fluid:
of air, initially at is passed through the shell side of a heat exchanger, with kghr of ammonia flowing through the tubes. There is no mixing of the streams.
The ammonia leaves the heat exchanger as a saturated vapour before entering a compressor, where
the pressure is raised to bar, producing a superheated vapour
The superheated vapour enters a poorly designed isobaric cooler that drops the temperature to
The resulting liquid stream is throttled through a valve, with a pressure gauge on the outlet reading
bar atmospheric pressure is bar The resulting twophase mixture is fed to the heat
exchanger to complete the cycle.
You are investigating the viability of replacing the valve with a reversible turbine in the hope of harvesting
some of the energy avalable when expanding the ammonia. A schematic of the original process is provided
below. If the heat exchanger operates adiabatically, what is the temperature of the exiting air? The next questions consider the case of replacing the valve with a turbine. You design the turbine in such a way that the ammonia still leaves the turbine at bar gauge. What is the entropy of the stream entering the turbine? Remembering the isentropic operation of the turbine, and using an energy balance over the unit, what is the quality of the stream leaving the turbine? If the heat exchanger operates adiabatically, what is the temperature of the exiting air? How much work could be extracted from the turbine remember the sign
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


