Question: Now we are ready to put everything together and actually calculate the instantaneous rate of this reation at ta 50 . seconds from the concentration
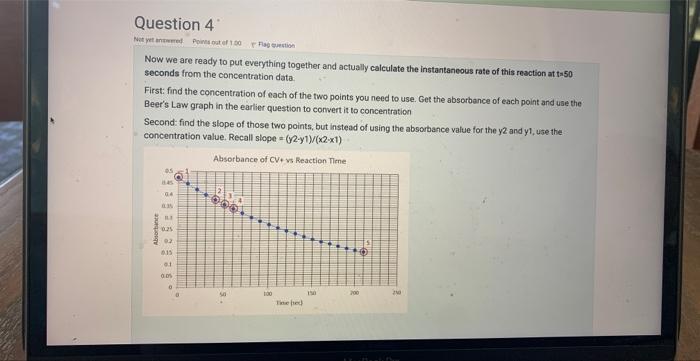
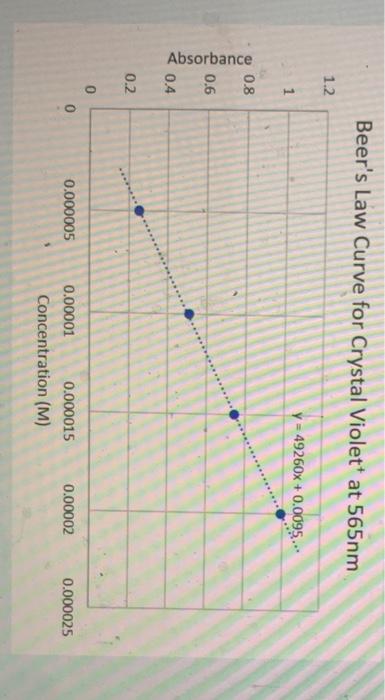
Now we are ready to put everything together and actually calculate the instantaneous rate of this reation at ta 50 . seconds from the concentration data. First: find the concentration of each of the fwo points you need to use. Get the absorbance of each point and use the Beer's Law graph in the earlier question to convert it to concentration Second: find the slope of those two points, but instead of using the absorbance value for the y2 and y1, use the concentration value. Recall slope =(y2y1)/(x2x1) Beer's Law Curve for Crystal Violet +at 565nm
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


