Question: number one options are A, C, F, E, D, B number two options are A, C, F, E, D, B number three options are the
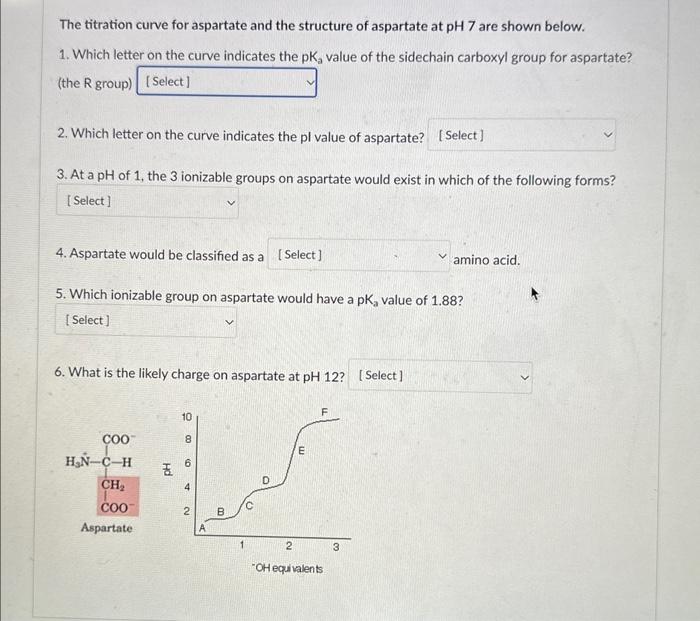
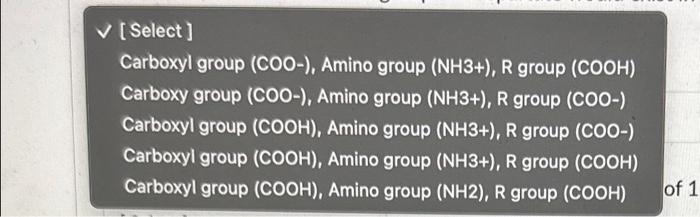
1. Which letter on the curve indicates the pKa value of the sidechain carboxyl group for aspartate? (the R group) 2. Which letter on the curve indicates the pl value of aspartate? 3. At a pH of 1 , the 3 ionizable groups on aspartate would exist in which of the following forms? 4. Aspartate would be classified as a amino acid. 5. Which ionizable group on aspartate would have a pKa value of 1.88 ? [Select] Carboxyl group (COO-), Amino group ( NH3+), R group ( COOH) Carboxy group (COO-), Amino group (NH3+), R group (COO-) Carboxyl group (COOH), Amino group (NH3+), R group (COO-) Carboxyl group (COOH), Amino group ( NH3+),R group ( COOH) Carboxyl group (COOH), Amino group ( NH2), R group (COOH)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


