Question: = = N.V N.V 2 OU c, =( = + NAV NV N.V a ln Z nRT2 ( az 4. We discussed in class how
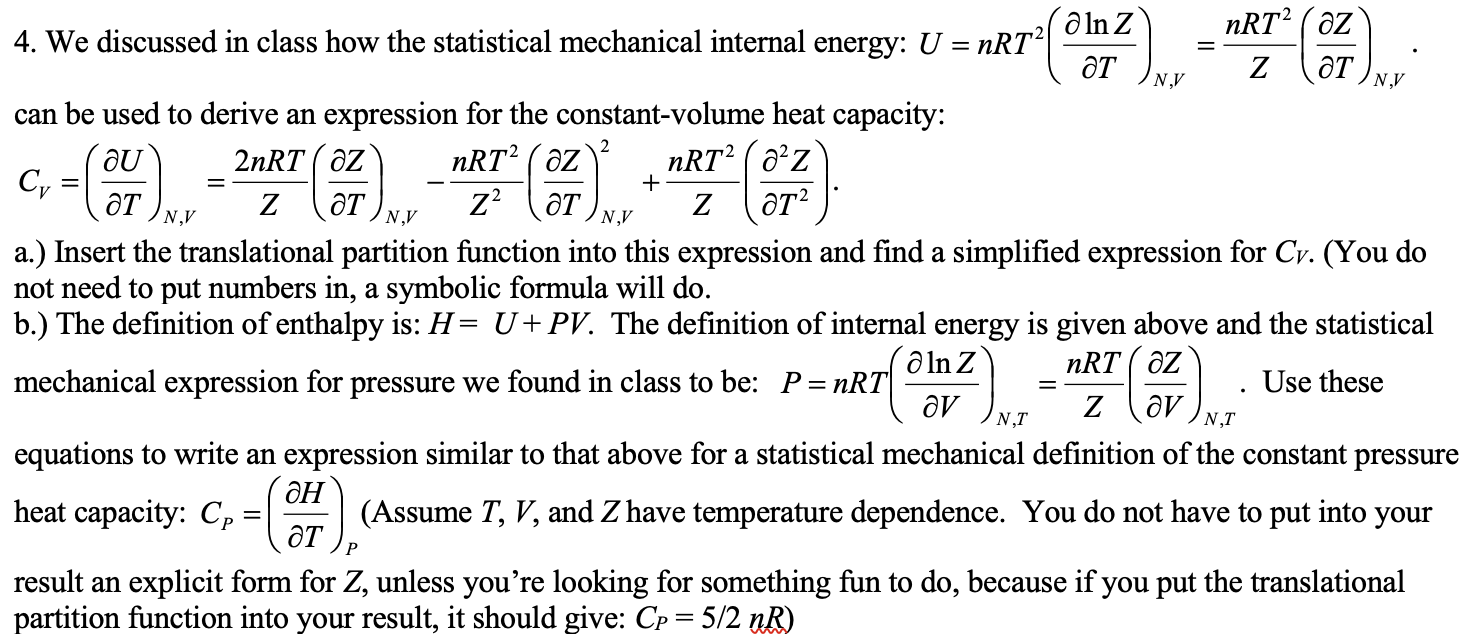
= = N.V N.V 2 OU c, =( = + NAV NV N.V a ln Z nRT2 ( az 4. We discussed in class how the statistical mechanical internal energy: U = nRT2 at Z at can be used to derive an expression for the constant-volume heat capacity: 2nRT az nRT2 ( az nRT2(a2z at Z aT Z? Z at2 a.) Insert the translational partition function into this expression and find a simplified expression for Cv. (You do not need to put numbers in, a symbolic formula will do. b.) The definition of enthalpy is: H= U+PV. The definition of internal energy is given above and the statistical a ln Z nRT AZ mechanical expression for pressure we found in class to be: P=nRT Use these av Z av equations to write an expression similar to that above for a statistical mechanical definition of the constant pressure aH heat capacity: Cp (Assume T, V, and Z have temperature dependence. You do not have to put into your result an explicit form for Z, unless you're looking for something fun to do, because if you put the translational partition function into your result, it should give: Cp = 5/2 nR) 2). N,T NT P
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


