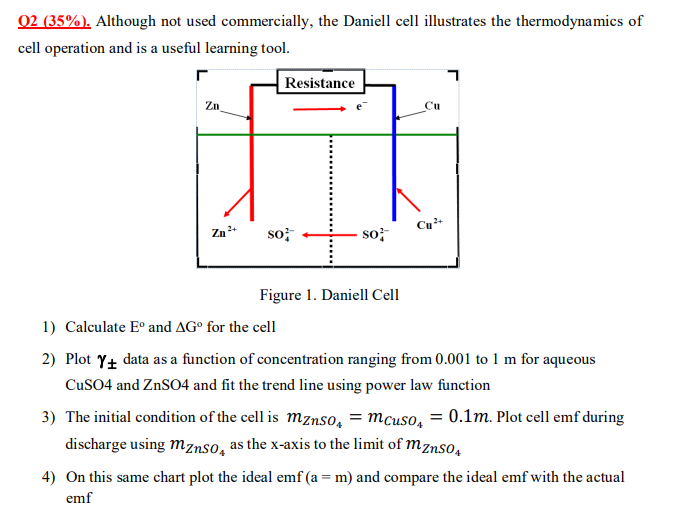
Question: O 2 ( 3 5 % ) . Although not used commercially, the Daniell cell illustrates the thermodynamics of cell operation and is a useful
O Although not used commercially, the Daniell cell illustrates the thermodynamics of
cell operation and is a useful learning tool.
Calculate and for the cell
Plot data as a function of concentration ranging from to for aqueous
CuSO and ZnSO and fit the trend line using power law function
The initial condition of the cell is Plot cell emf during
discharge using as the xaxis to the limit of
On this same chart plot the ideal emf and compare the ideal emf with the actual
emf
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


