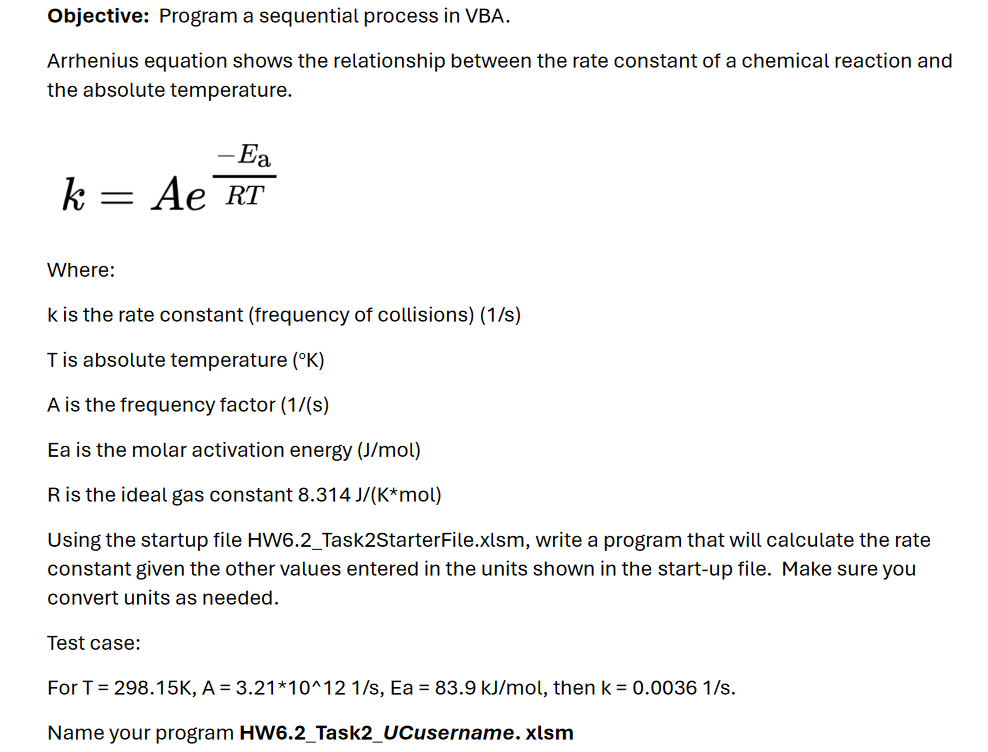
Question: Objective: Program a sequential process in VBA. Arrhenius equation shows the relationship between the rate constant of a chemical reaction and the absolute temperature. k
Objective: Program a sequential process in VBA.
Arrhenius equation shows the relationship between the rate constant of a chemical reaction and
the absolute temperature.
Where:
is the rate constant frequency of collisionss
T is absolute temperature
A is the frequency factor
Ea is the molar activation energy
is the ideal gas constant
Using the startup file HWTaskStarterFile.xlsm write a program that will calculate the rate
constant given the other values entered in the units shown in the startup file. Make sure you
convert units as needed.
Test case:
For then
Name your program HWTaskUCusername. xlsm
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


