Question: +OH Which side of this equilibrium is favored? Reactant side because OHis the less stable base Reactant side because OHis the more stable base Product
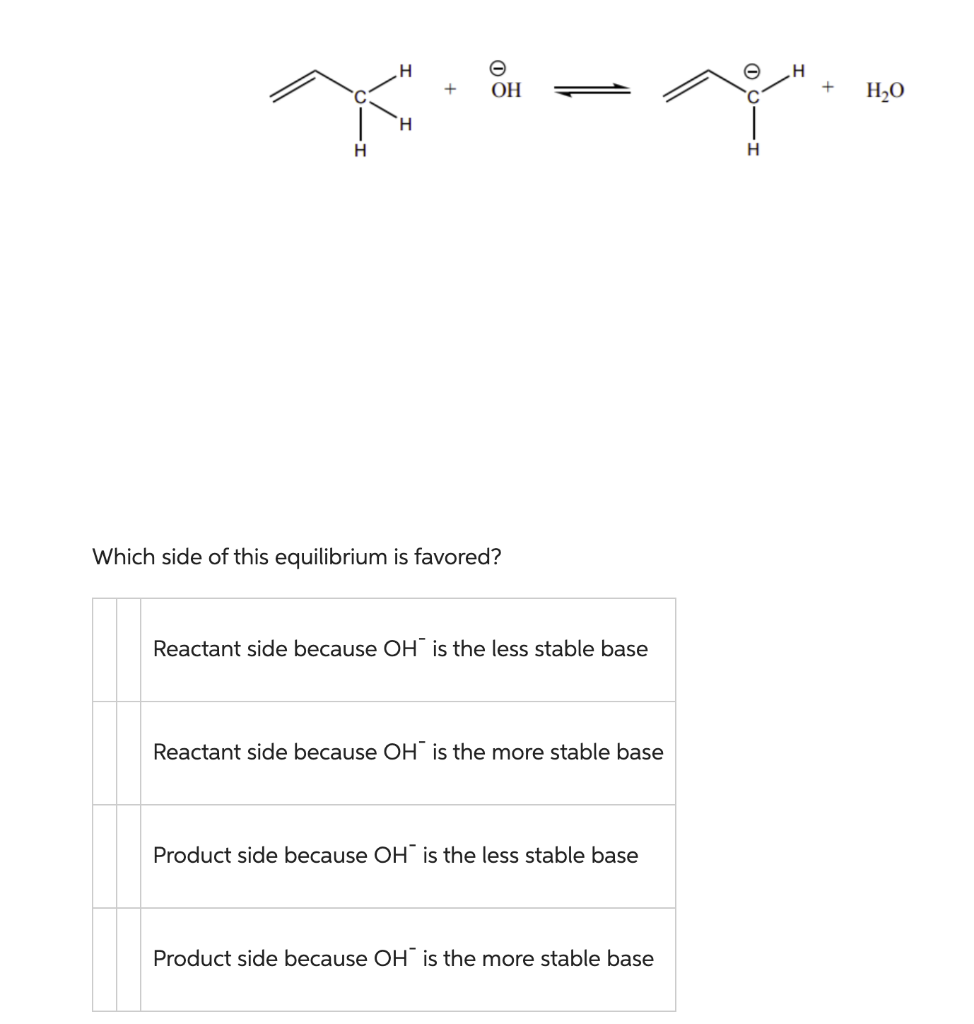
+OH Which side of this equilibrium is favored? Reactant side because OHis the less stable base Reactant side because OHis the more stable base Product side because OHis the less stable base Product side because OHis the more stable base
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


