Question: On a 0.146 mm diameter column with a 0.935 m thick stationary phase, an unretained solute passes through the column in 1.23 min, whereas
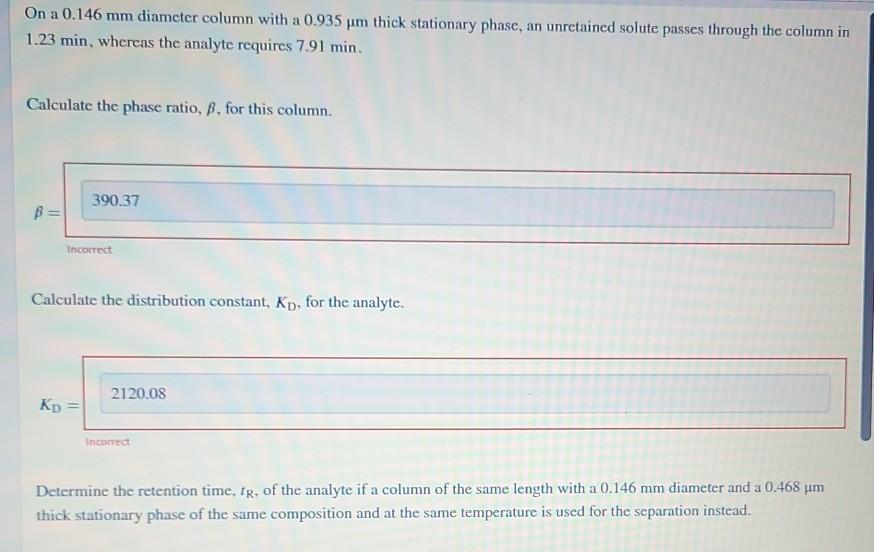

On a 0.146 mm diameter column with a 0.935 m thick stationary phase, an unretained solute passes through the column in 1.23 min, whereas the analyte requires 7.91 min. Calculate the phase ratio, , for this column. B 390.37 KD Incorrect Calculate the distribution constant, Kp, for the analyte. 2120.08 Incorrect Determine the retention time, IR, of the analyte if a column of the same length with a 0.146 mm diameter and a 0.468 m thick stationary phase of the same composition and at the same temperature is used for the separation instead. Determine the retention time, fg, of the analyte if a column of the same length with a 0.146 mm diameter and a 0.468 m thick stationary phase of the same composition and at the same temperature is used for the separation instead. IR = 10.87 Incorrect min
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
636029c34cbcb_233628.pdf
180 KBs PDF File
636029c34cbcb_233628.docx
120 KBs Word File


