Question: One process for removing CO2 from the atmosphere is to convert CO2 to carbonate (CO32) and then precipitate it as calcite (CaCO3). The following equilibria
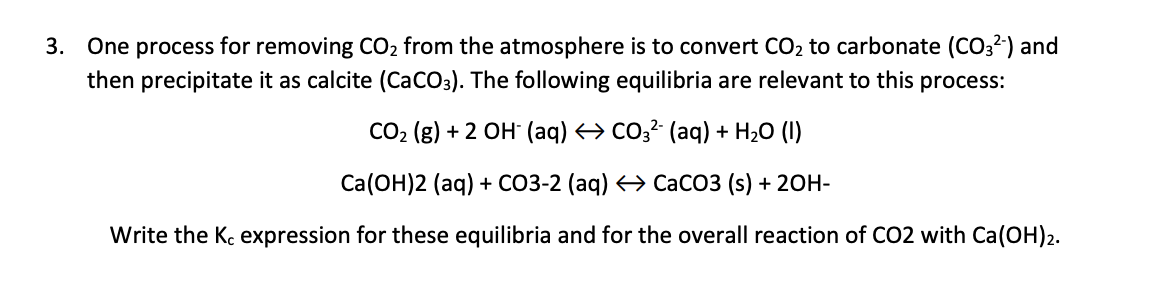
One process for removing CO2 from the atmosphere is to convert CO2 to carbonate (CO32) and then precipitate it as calcite (CaCO3). The following equilibria are relevant to this process: CO2(g)+2OH(aq)CO32(aq)+H2O(l)Ca(OH)2(aq)+CO2(aq)CaCO3(s)+2OH Write the Kc expression for these equilibria and for the overall reaction of CO2 with Ca(OH)2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


