Question: Question 3 Whether you meet the desulfurization criterion specified in Q.2. or not, you proceed with process design assuming all the sulfur is removed through
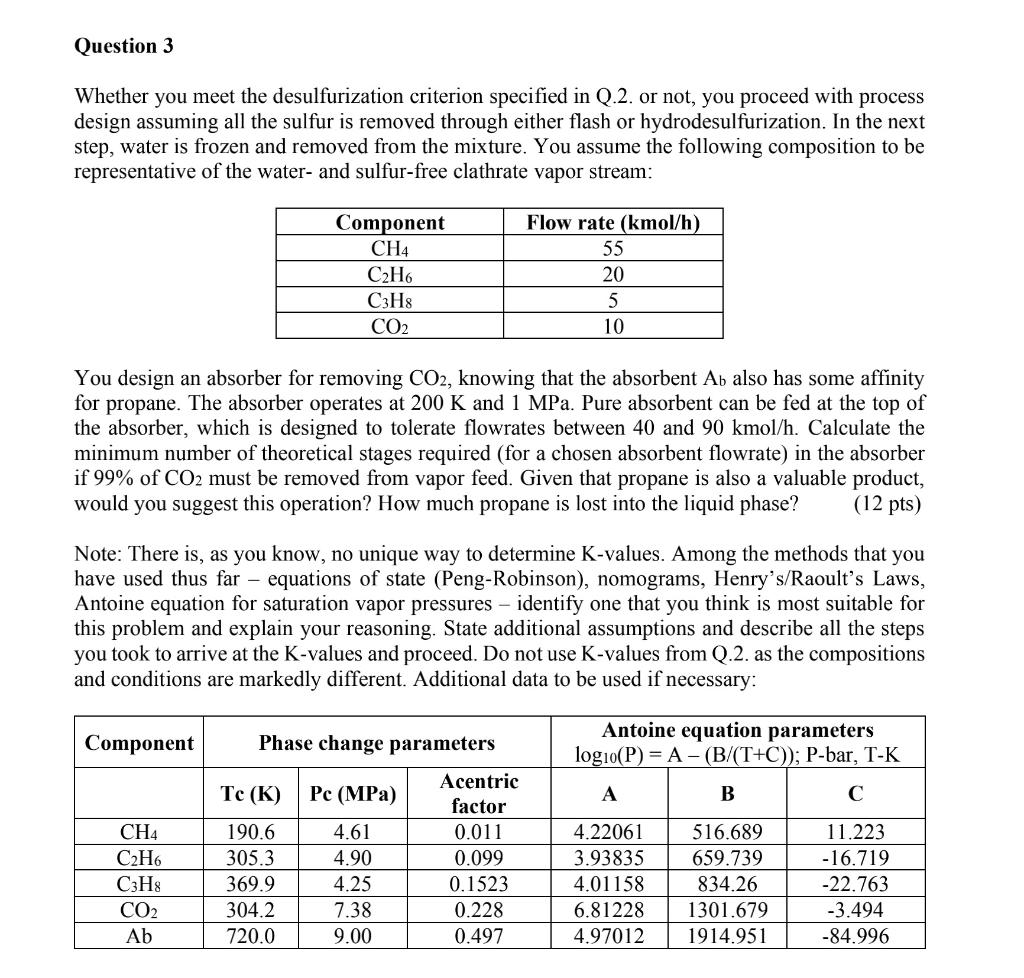

Question 3 Whether you meet the desulfurization criterion specified in Q.2. or not, you proceed with process design assuming all the sulfur is removed through either flash or hydrodesulfurization. In the next step, water is frozen and removed from the mixture. You assume the following composition to be representative of the water- and sulfur-free clathrate vapor stream: Component CH4 C2H6 C3H8 CO2 Flow rate (kmol/h) 55 20 5 10 You design an absorber for removing CO2, knowing that the absorbent Ab also has some affinity for propane. The absorber operates at 200 K and 1 MPa. Pure absorbent can be fed at the top of the absorber, which is designed to tolerate flowrates between 40 and 90 kmol/h. Calculate the minimum number of theoretical stages required (for a chosen absorbent flowrate) in the absorber if 99% of CO2 must be removed from vapor feed. Given that propane is also a valuable product, would you suggest this operation? How much propane is lost into the liquid phase? (12 pts) Note: There is, as you know, no unique way to determine K-values. Among the methods that you have used thus far equations of state (Peng-Robinson), nomograms, Henry's/Raoult's Laws, Antoine equation for saturation vapor pressures - identify one that you think is most suitable for this problem and explain your reasoning. State additional assumptions and describe all the steps you took to arrive at the K-values and proceed. Do not use K-values from Q.2. as the compositions and conditions are markedly different. Additional data to be used if necessary: Component Antoine equation parameters logio(P) = A - (B/(T+C)); P-bar, T-K A B CH4 C2H6 C3H8 CO2 Ab Phase change parameters Acentric Tc (K) Pc (MPa) factor 190.6 4.61 0.011 305.3 4.90 0.099 369.9 4.25 0.1523 304.2 7.38 0.228 720.0 9.00 0.497 4.22061 3.93835 4.01158 6.81228 4.97012 516.689 659.739 834.26 1301.679 1914.951 11.223 - 16.719 -22.763 -3.494 -84.996 Question 4 To determine the number of actual stages, you set out to estimate overall stage efficiency. The average molecular weight in the liquid phase in the column is 62 g/mol, viscosity is 18 CP, and density is 1.11 g/cm3. Calculate the overall stage efficiency and therefore the number of actual stages. (4 pts)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


