Question: Only c) and d) only c) and d), thanks. 1) An experiment was conducted to estimate the reaction order, the reaction rate constant, and the
Only c) and d)
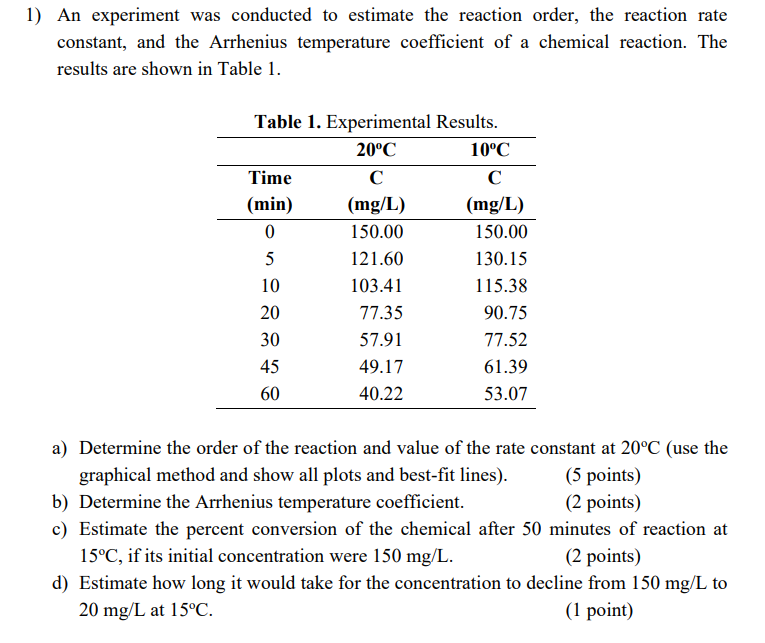
only c) and d), thanks.
1) An experiment was conducted to estimate the reaction order, the reaction rate constant, and the Arrhenius temperature coefficient of a chemical reaction. The results are shown in Table 1. a) Determine the order of the reaction and value of the rate constant at 20C (use the graphical method and show all plots and best-fit lines). (5 points) b) Determine the Arrhenius temperature coefficient. (2 points) c) Estimate the percent conversion of the chemical after 50 minutes of reaction at 15C, if its initial concentration were 150mg/L. (2 points) d) Estimate how long it would take for the concentration to decline from 150mg/L to 20mg/L at 15C. (1 point)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


