Question: only solve for the part that is not filled out. which is the system temperature and pressure section of the question. do not solve for
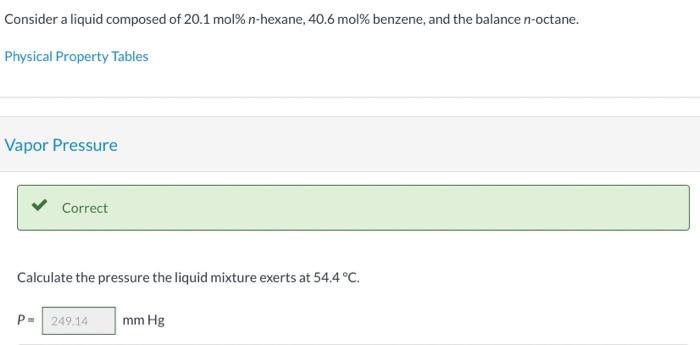
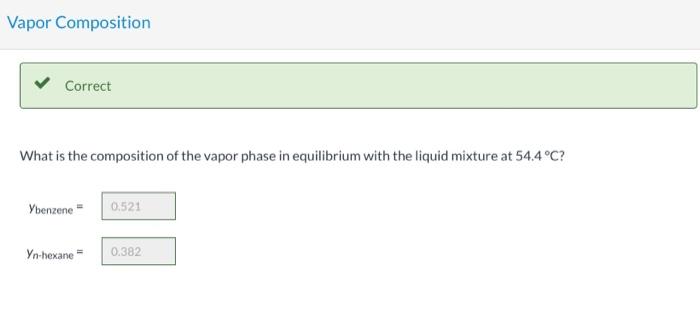
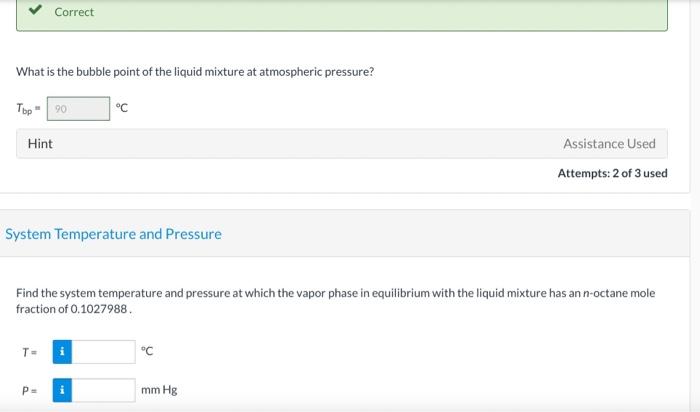
Consider a liquid composed of 20.1 mol% n-hexane, 40.6 mol% benzene, and the balance n-octane. Physical Property Tables Vapor Pressure Correct Calculate the pressure the liquid mixture exerts at 54.4C. P249.14 mm Hg Vapor Composition Correct What is the composition of the vapor phase in equilibrium with the liquid mixture at 54.4C? Ybenzene 0,521 Yn-hexane 0.382 Correct What is the bubble point of the liquid mixture at atmospheric pressure? Top 20 C Hint Assistance Used Attempts: 2 of 3 used System Temperature and Pressure Find the system temperature and pressure at which the vapor phase in equilibrium with the liquid mixture has an n-octane mole fraction of 0.1027988 T- i C PE mm Hg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


