Question: Only solve question 2 please! Using the answer from question 1 in calculations when needed. 1. Using the enthalpies of formation given below, calculate Hrxn
 Only solve question 2 please! Using the answer from question 1 in calculations when needed.
Only solve question 2 please! Using the answer from question 1 in calculations when needed.
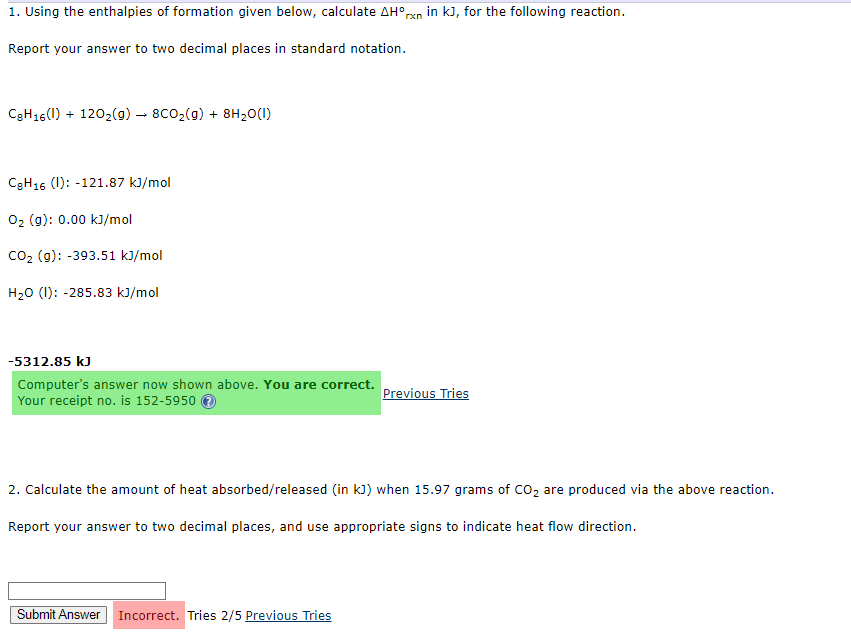
1. Using the enthalpies of formation given below, calculate Hrxn in kJ, for the following reaction. Report your answer to two decimal places in standard notation. C8H16(I)+12O2(g)8CO2(g)+8H2O(I) C8H16(I):121.87kJ/molO2(g):0.00kJ/molCO2(g):393.51kJ/molH2O(I):285.83kJ/mol -5312.85 kJ Computer's answer now shown above. You are correct. Your receipt no. is 1525950 (7) 2. Calculate the amount of heat absorbed/released (in kJ ) when 15.97 grams of CO2 are produced via the above reaction. Report your answer to two decimal places, and use appropriate signs to indicate heat flow direction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


