Question: Only solve question b, the answer should be 4084 kW 6. Ethylbenzene (C8H10) is commercially produced through the direct addition reaction between ethylene (C2H4) and
Only solve question b, the answer should be 4084 kW
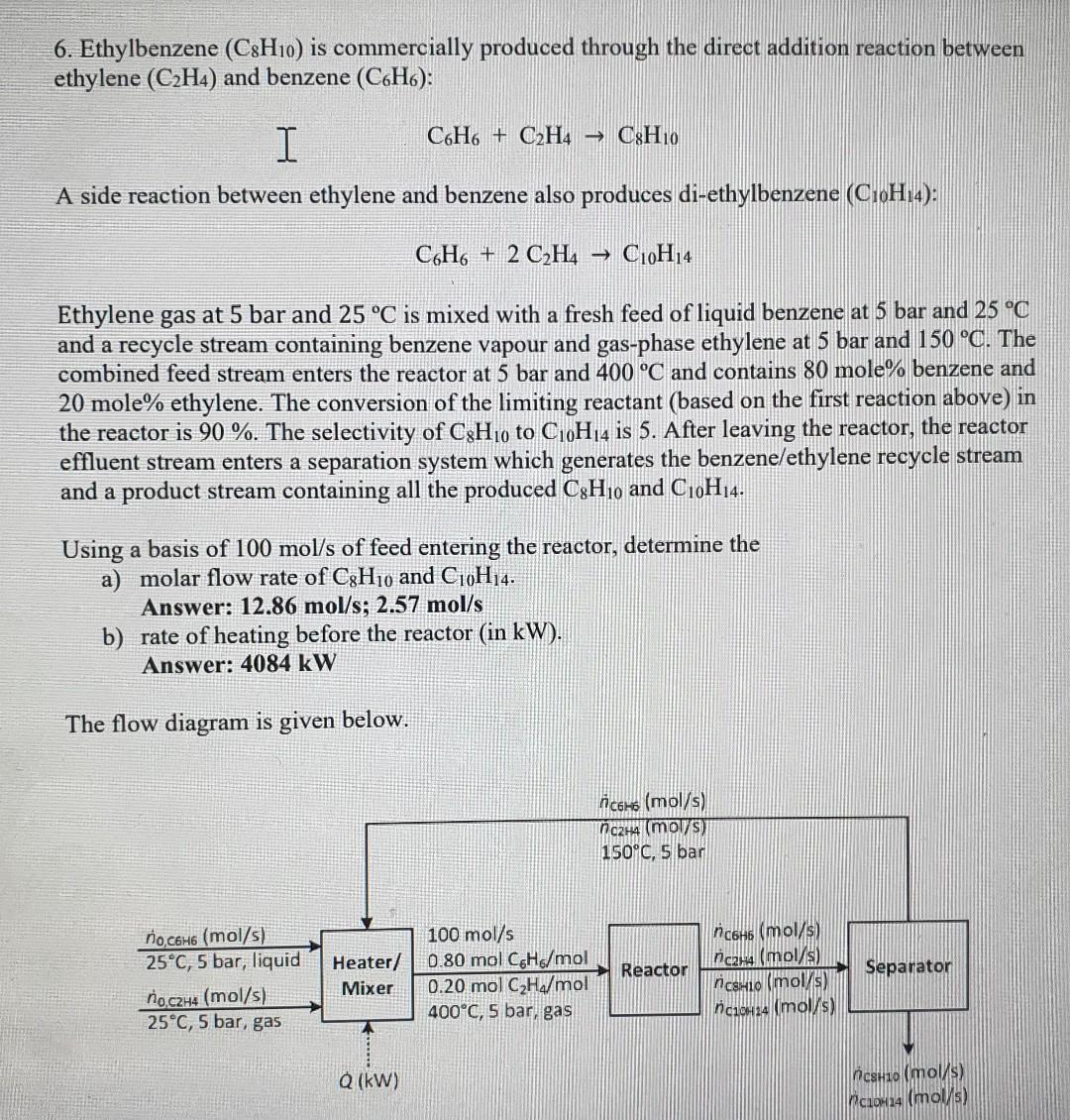
6. Ethylbenzene (C8H10) is commercially produced through the direct addition reaction between ethylene (C2H4) and benzene (C6H6) : IC6H6+C2H4C8H10 A side reaction between ethylene and benzene also produces di-ethylbenzene (C10H14) : C6H6+2C2H4C10H14 Ethylene gas at 5 bar and 25C is mixed with a fresh feed of liquid benzene at 5 bar and 25C and a recycle stream containing benzene vapour and gas-phase ethylene at 5 bar and 150C. The combined feed stream enters the reactor at 5 bar and 400C and contains 80 mole\% benzene and 20 mole\% ethylene. The conversion of the limiting reactant (based on the first reaction above) in the reactor is 90%. The selectivity of C8H10 to C10H14 is 5 . After leaving the reactor, the reactor effluent stream enters a separation system which generates the benzene/ethylene recycle stream and a product stream containing all the produced C8H10 and C10H14. Using a basis of 100mol/s of feed entering the reactor, determine the a) molar flow rate of C8H10 and C10H14. Answer: 12.86mol/s;2.57mol/s b) rate of heating before the reactor (in kW ). Answer: 4084kW The flow diagram is given below
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


