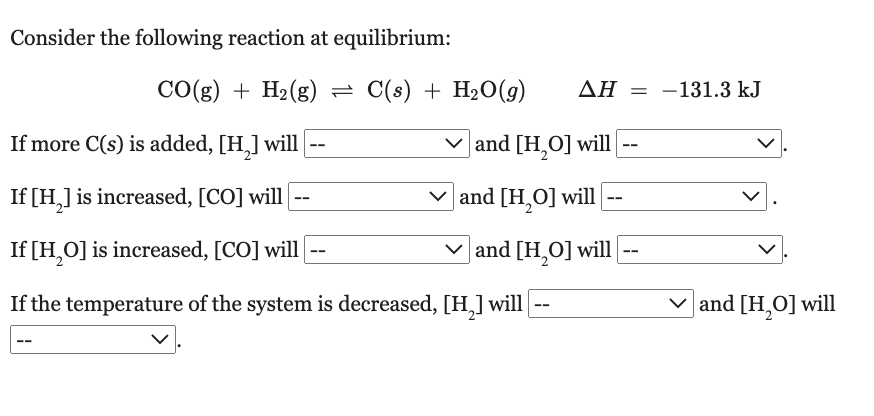
Question: options are decrease, increase, stay the same Consider the following reaction at equilibrium: CO(g)+H2(g)C(s)+H2O(g)H=131.3kJ If more C(s) is added, [H2] will and [H2O] will If
options are decrease, increase, stay the same
Consider the following reaction at equilibrium: CO(g)+H2(g)C(s)+H2O(g)H=131.3kJ If more C(s) is added, [H2] will and [H2O] will If [H2] is increased, [CO] will and [H2O] will If [H2O] is increased, [CO] will and [H2O] will If the temperature of the system is decreased, [H2] will and [H2O] will
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


