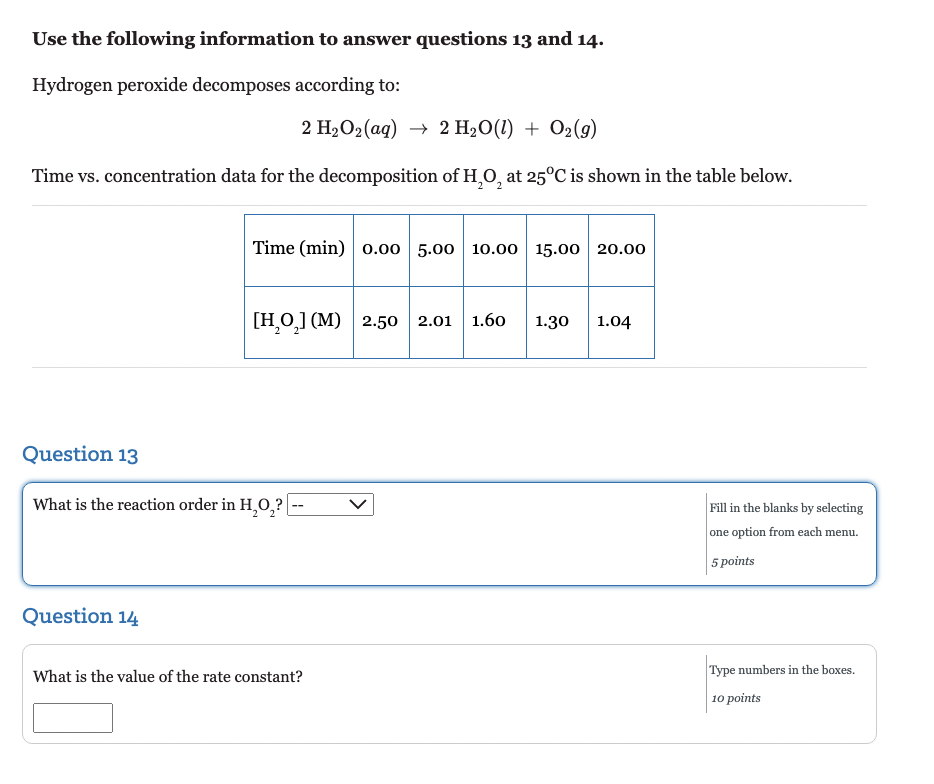
Question: options for first one are: zero, first, second Use the following information to answer questions 13 and 14 . Hydrogen peroxide decomposes according to: 2H2O2(aq)2H2O(l)+O2(g)
options for first one are: zero, first, second
Use the following information to answer questions 13 and 14 . Hydrogen peroxide decomposes according to: 2H2O2(aq)2H2O(l)+O2(g) Time vs. concentration data for the decomposition of H2O2 at 25C is shown in the table below. Question 13 What is the reaction order in H2O2 ? Fill in the blanks by selecting one option from each menu. 5 points Question 14 What is the value of the rate constant? Type numbers in the boxes
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


