Question: options for first one: options for second one: A and B react according to: Fill in the blanks by selecting A+2Bproducts one option from each
options for first one: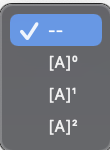
options for second one: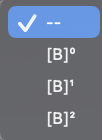
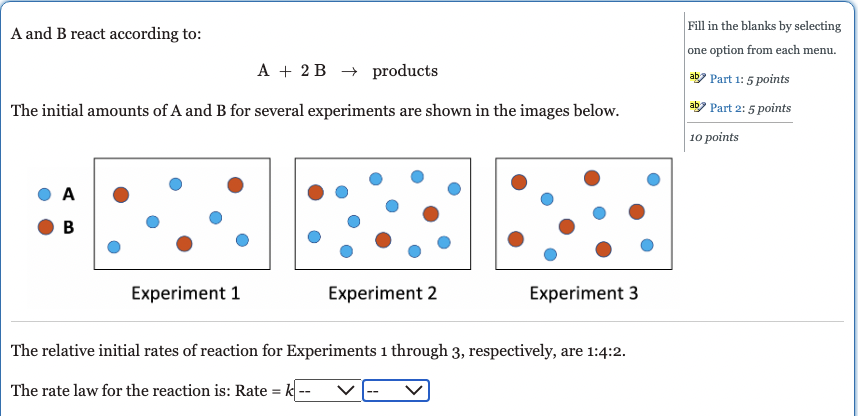
A and B react according to: Fill in the blanks by selecting A+2Bproducts one option from each menu. abs Part 1: 5 points The initial amounts of A and B for several experiments are shown in the images below. 10pointsabPart2:5points A B Experiment 1 Experiment 2 Experiment 3 The relative initial rates of reaction for Experiments 1 through 3 , respectively, are 1:4:2. The rate law for the reaction is: Rate =k [A] [A]1 [A]2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


