Question: OPTIONS: Type of solid formed- Molecular, atomic, ionic Force holding the solid together - dispersion, dipole, hydrogen bonding, metallic bonding, ionic bonding, covalent bonding. melting
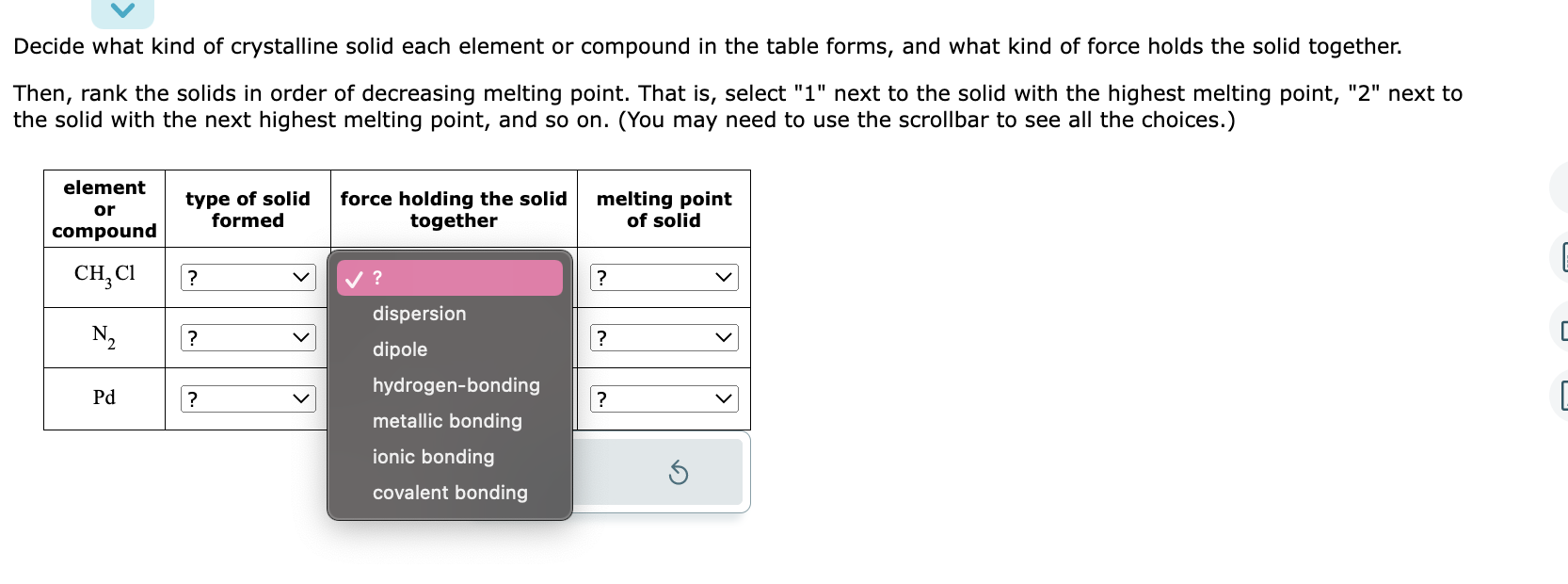
OPTIONS:
Type of solid formed- Molecular, atomic, ionic
Force holding the solid together - dispersion, dipole, hydrogen bonding, metallic bonding, ionic bonding, covalent bonding.
melting point of solid - 1, 2, 3
Decide what kind of crystalline solid each element or compound in the table forms, and what kind of force holds the solid together. Then, rank the solids in order of decreasing melting point. That is, select " 1 " next to the solid with the highest melting point, "2" next to the solid with the next highest melting point, and so on. (You may need to use the scrollbar to see all the choices.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


