Question: or in symbols, Q=(m)(c )(AT) When using this equation, always write AT as a positive quantity. When the hot metal is placed in the water,
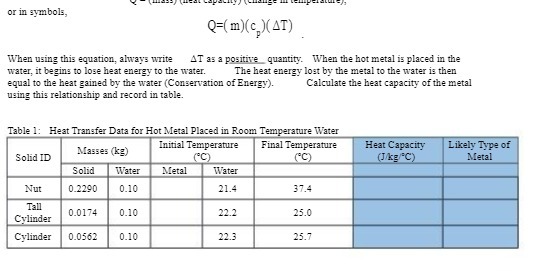
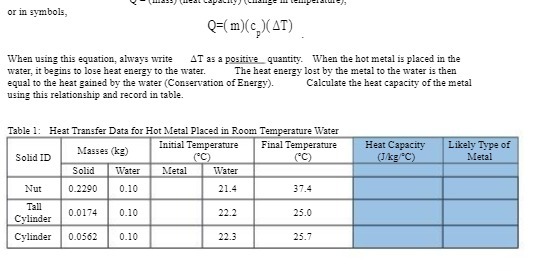
or in symbols, Q=(m)(c )(AT) When using this equation, always write AT as a positive quantity. When the hot metal is placed in the water, it begins to lose heat energy to the water. The heat energy lost by the metal to the water is them equal to the heat gained by the water (Conservation of Energy). Calculate the heat capacity of the metal using this relationship and record in table. Table 1: Heat Transfer Data for Hot Metal Placed in Room Temperature Water Masses (kg) Initial Temperature Final Temperature Heat Capacity Likely Type of Solid ID (JAKE C) Metal Solid Water Metal Water Nut 0.2290 0.10 21.4 37.4 Tall 0.0174 0.10 22.2 25.0 Cylinder Cylinder 0_0562 0.10 22.3 25.7
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


