Question: Oxalic acid dihydrate is a solid, diprotic acid that can be used in the laboratory as a primary standard. Its formula is H2C2O42H2O. A student
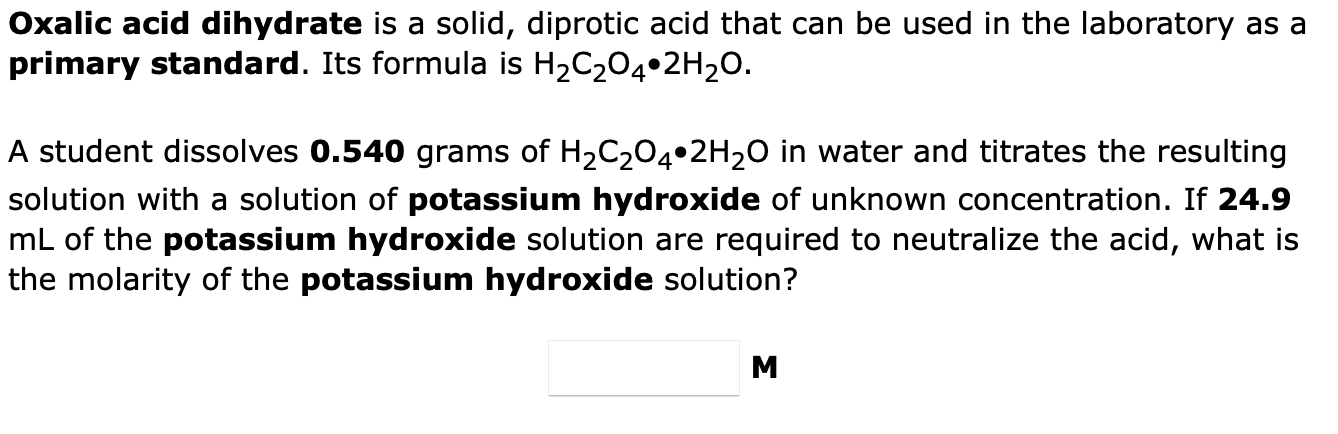
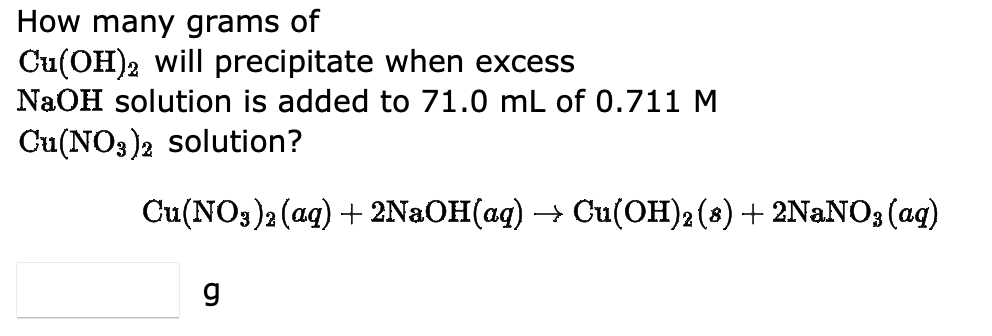
Oxalic acid dihydrate is a solid, diprotic acid that can be used in the laboratory as a primary standard. Its formula is H2C2O42H2O. A student dissolves 0.540 grams of H2C2O42H2O in water and titrates the resulting solution with a solution of potassium hydroxide of unknown concentration. If 24.9 mL of the potassium hydroxide solution are required to neutralize the acid, what is the molarity of the potassium hydroxide solution? M How many grams of Cu(OH)2 will precipitate when excess NaOH solution is added to 71.0mL of 0.711M Cu(NO3)2 solution? Cu(NO3)2(aq)+2NaOH(aq)Cu(OH)2(s)+2NaNO3(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


