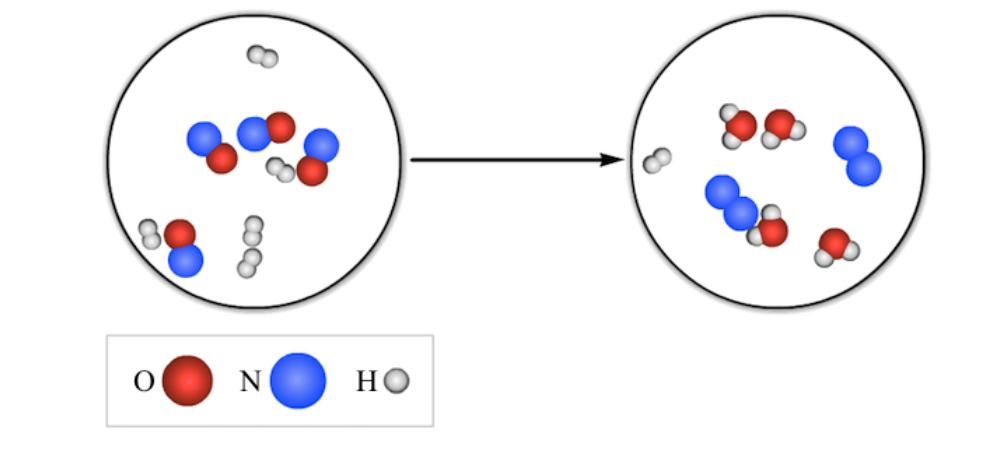
Question: Oxygen is represented by a red sphere. Nitrogen is represented by a blue sphere. Hydrogen is represented by a white sphere. In the reactants there
Oxygen is represented by a red sphere. Nitrogen is represented by a blue sphere. Hydrogen is represented by a white sphere. In the reactants there are five molecules that contain two white spheres and four molecules that contains one red and one blue sphere. In the products there are two molecules that contain two blue spheres, four molecules that contain one red and two white spheres, and one molecule that contains two white spheres. What is the chemical formula for the limiting reactant in the reaction shown?

N
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it
To determine the limiting reactant lets identify the molecules based on the sphere colors and count ... View full answer

Get step-by-step solutions from verified subject matter experts


