Question: Ozone (O(g)) can adsorb on a metallic surface while disintegrating into O2 and O adsorbed (two adjacent sites,) Decomposition into three adsorbed oxygen atoms (three
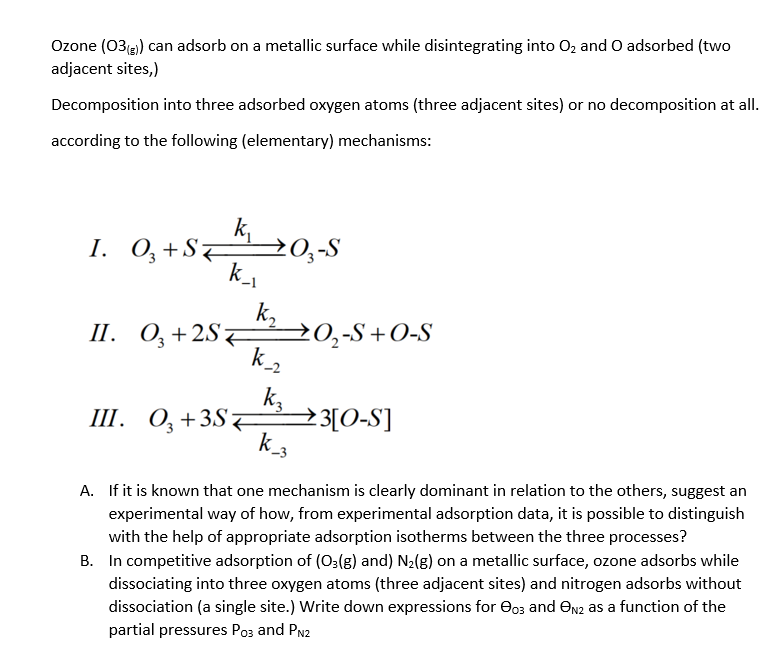
Ozone (O(g)) can adsorb on a metallic surface while disintegrating into O2 and O adsorbed (two adjacent sites,) Decomposition into three adsorbed oxygen atoms (three adjacent sites) or no decomposition at all. according to the following (elementary) mechanisms: I. O3+Sk1k1O3S II. O3+2Sk2k2O2S+OS III. O3+3Sk3k33[OS] A. If it is known that one mechanism is clearly dominant in relation to the others, suggest an experimental way of how, from experimental adsorption data, it is possible to distinguish with the help of appropriate adsorption isotherms between the three processes? B. In competitive adsorption of (O3(g) and) N2(g) on a metallic surface, ozone adsorbs while dissociating into three oxygen atoms (three adjacent sites) and nitrogen adsorbs without dissociation (a single site.) Write down expressions for O and N2 as a function of the partial pressures P03 and PN2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


