Question: P 1 0 - 4 B B Consider the catalytic reaction as a function of the initial partial pressures 2 A B + C The
P Consider the catalytic reaction as a function of the initial partial pressures
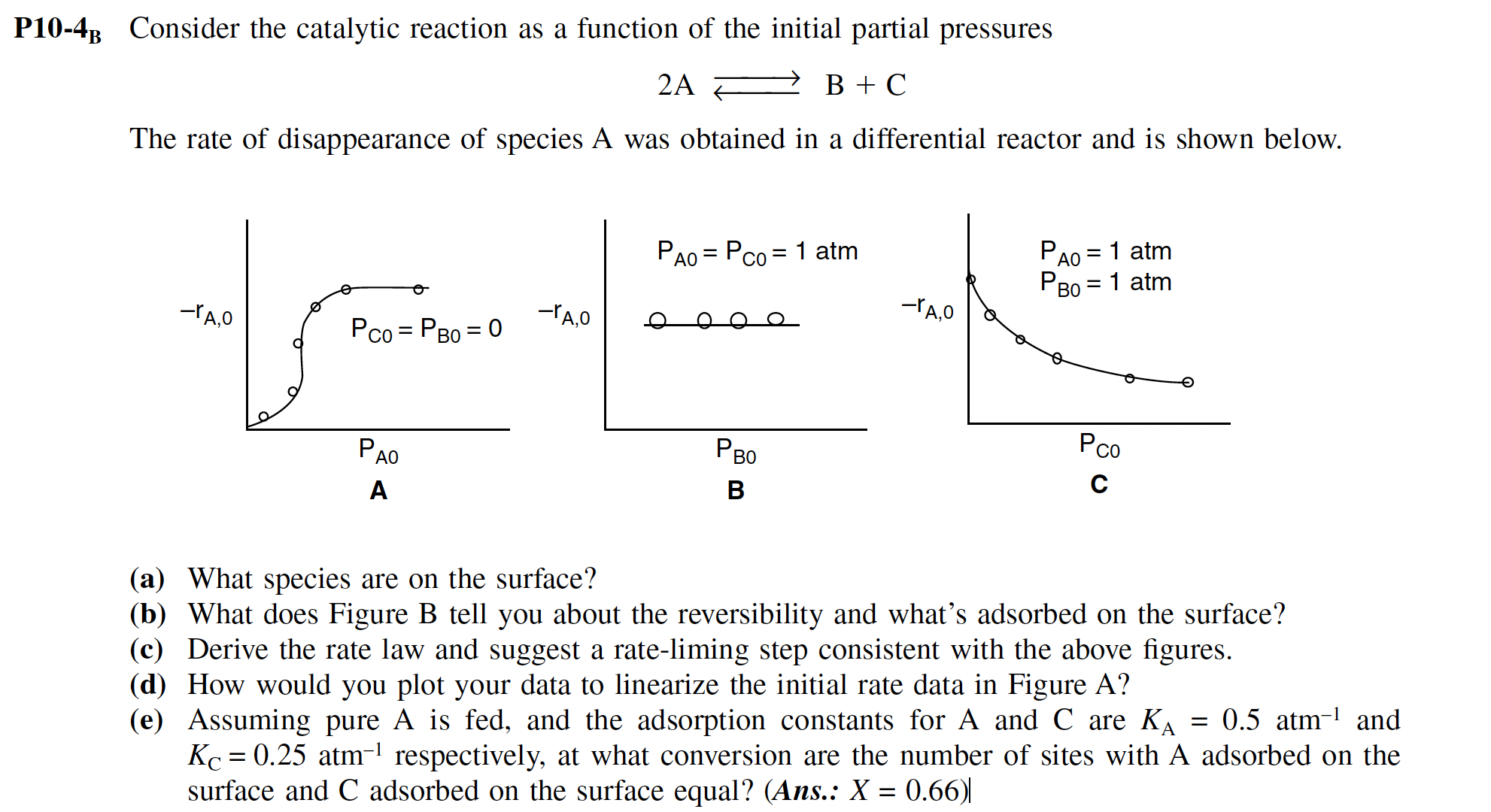
The rate of disappearance of species A was obtained in a differential reactor and is shown below.
a What species are on the surface?
b What does Figure B tell you about the reversibility and what's adsorbed on the surface?
c Derive the rate law and suggest a rateliming step consistent with the above figures.
d How would you plot your data to linearize the initial rate data in Figure A
e Assuming pure is fed, and the adsorption constants for A and are and
respectively, at what conversion are the number of sites with A adsorbed on the
surface and adsorbed on the surface equal? Ans:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


