Question: P 2 - 2 A ( a ) Revisit the data in Table 2 - 1 Raw Data and calculate the batch reactor ( BR
P A a Revisit the data in Table Raw Data and calculate the batch reactor BR times to achieve and conversion when moles of A are charged to a reactor.
b Revisit Example through How would your answers change if the flow rate, were reduced to one quarter? If it were tripled? What conversion can be achieved in a and in a CSTR
c Revisit Example Being a company about to go bankrupt, you can only afford a CSTR What conversion can you achieve?
d Revisit Example What conversion could you achieve if you could convince your boss, Dr Pennypincher, to spend more money to buy a PFR to attach to a CSTR
e Revisit Example How would your answers change if the two CSTRs one and the other were placed in parallel with the flow, divided equally between the reactors?
e Revisit Example What is the worst possible way to arrange the two CSTRs and one PFR What would be the reactor volumes if the two intermediate conversion were changed to and respectively? What would be the conversions, and if all the reactors had the same volume of and were placed in the same order?
g Revisit Example If the term is seconds for conversion, how much fluid can you process in a reactor?
P B You have two CSTRs and two PFRs each with a volume of Use Figure b on page to calculate the conversion for each of the reactors in the following arrangements.
a Two CSTRs in series. Ans:
b Two PFRs in series.
c Two CSTRs in parallel with the feed, divided equally between the two reactors.
d Two PFRs in parallel with the feed divided equally between the two reactors.
e A PFR followed by a CSTR
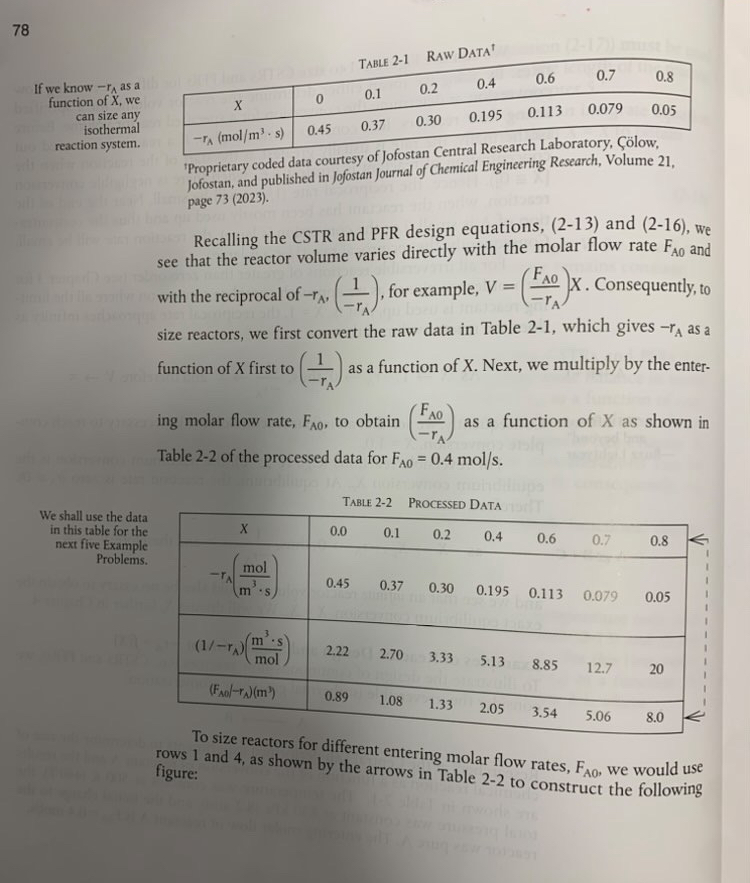
If we know as a function of we can size any isothermal reaction system.
We shall use the data in this table for the next five Example Problems.
'Proprietary coded data courtesy of Jofostan Central Research Laboratory, low Jofostan, and published in Jofostan Journal of Chemical Engineering Research, Volume page
Recalling the CSTR and PFR design equations, and we see that the reactor volume varies directly with the molar flow rate and with the reciprocal of for example, Consequently, to size reactors, we first convert the raw data in Table which gives as a function of first to as a function of Next, we multiply by the entering molar flow rate, to obtain as a function of as shown in Table of the processed data for
TABLE PRocessed Data
table
To size reactors for different entering molar flow rates, we would use rows and as shown by the arrows in Table to construct the following figure:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


