1. Revisit the data in Table 2-1 Raw Data and calculate the batch reactor (BR) times to...
Question:
1.
Revisit the data in Table 2-1 Raw Data and calculate the batch reactor (BR) times to achieve 10%, 50%, and 80% conversion when 100 moles of A are charged to a 400 dm3 reactor.
Table 2-1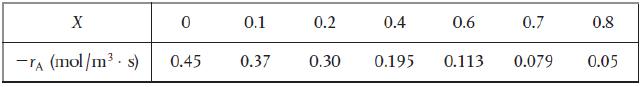
2.
Revisit Examples 2-1 through 2-3. How would your answers change if the flow rate, FA0, were cut in half? If it were doubled? What conversion can be achieved in a 4.5 m3 PFR and in a 4.5 m3 CSTR?
Examples 2-1
The reaction described by the data in Table 2-2 A → B is to be carried out in a CSTR.
Table 2-2
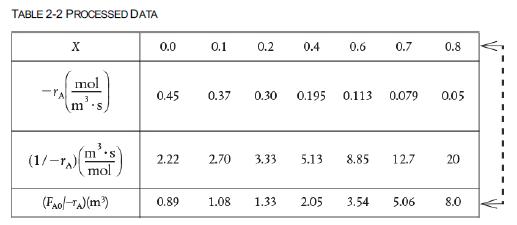
Species A enters the reactor at a molar flow rate of FA0=0.4mo1s, which is the flow rate used to construct the Levenspiel plot in Figure 2-2(b).
Figure 2-2(b)
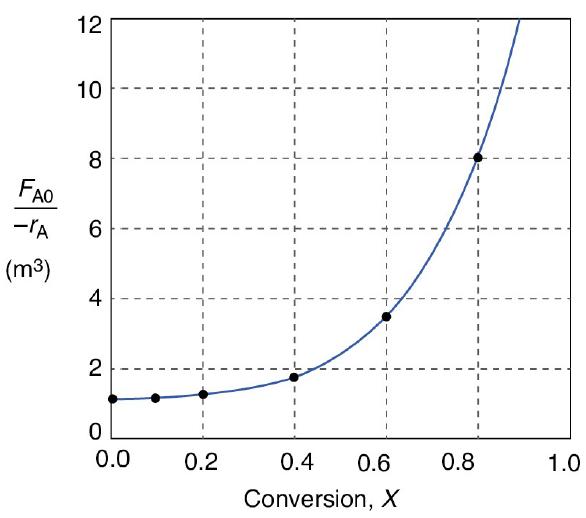 1. Using the data in either Table 2-2 or Figure 2-2(b), calculate the volume necessary to achieve 80% conversion in a CSTR.
1. Using the data in either Table 2-2 or Figure 2-2(b), calculate the volume necessary to achieve 80% conversion in a CSTR.
2. Shade the area in Figure 2-2(b) that would give the CSTR volume necessary to achieve 80% conversion.
Examples 2-2
The reaction described by the data in Tables 2-1 and 2-2 is to be carried out in a PFR. The entering molar flow rate of A is again, FA0 = 0.4 mol/s.
1. First, use one of the integration formulas to determine the PFR reactor volume necessary to achieve 80% conversion.
2. Next, shade the area in Figure 2-2(b) that would give the PFR volume necessary to achieve 80% conversion.
3. Finally, make a qualitative sketch of the conversion, X, and the rate of reaction, –rA, down the length (volume) of the reactor.
Tables 2-1
Examples 2-3
Compare the volumes of a CSTR and a PFR required for the same conversion using the data in Figure 2-2(b). Which reactor would require the smaller volume to achieve a conversion of 80%: a CSTR or a PFR? The entering molar flow rate and the feed conditions are the same in both cases.
Figure 2-2(b)

3.
Revisit Example 2-2. Being a company about to go bankrupt, you can only afford a 2.5 m3 CSTR. What conversion can you achieve?
The reaction described by the data in Tables 2-1 and 2-2 is to be carried out in a PFR. The entering molar flow rate of A is again, FA0 = 0.4 mol/s.
1. First, use one of the integration formulas to determine the PFR reactor volume necessary to achieve 80% conversion.
2. Next, shade the area in Figure 2-2(b) that would give the PFR volume necessary to achieve 80% conversion.
3. Finally, make a qualitative sketch of the conversion, X, and the rate of reaction, –rA, down the length (volume) of the reactor.
Tables 2-1
4.
Revisit Example 2-3. What conversion could you achieve if you could convince your boss, Dr. Pennypincher, to spend more money to buy a 1.0 m3 PFR to attach to a 2.40 CSTR?
Figure 2-2(b)

5.
Revisit Example 2-4. How would your answers change if the two CSTRs (one 0.82 m3 and the other 3.2 m3) were placed in parallel with the flow, FA0, divided equally between the reactors?
Example 2-4
For the two CSTRs in series, 40% conversion is achieved in the first reactor. What is the volume of each of the two reactors necessary to achieve 80% overall conversion of the entering species A?
6.
Revisit Example 2-5.
(1) What is the worst possible way to arrange the two CSTRs and one PFR?
(2) What would be the reactor volumes if the two intermediate conversions were changed to 20% and 50%, respectively?
(3) What would be the conversions, X1, X2, and X3, if all the reactors had the same volume of 100 dm3 and were placed in the same order?
Example 2-5
The isomerization of butane
7.
Revisit Example 2-6. If the term CA0∫0XdX-rA is 2 seconds for 80% conversion, how much fluid (m3/min) can you process in a 3 m3 reactor?
Example 2-6
Calculate the space time, τ, and space velocities for the reactor in Examples 2-1 and 2-3 for an entering volumetric flow rate of 2 dm3/s.
Examples 2-1
The reaction described by the data in Table 2-2 A → B is to be carried out in a CSTR.
Table 2-2

Example 2-3
Compare the volumes of a CSTR and a PFR required for the same conversion using the data in Figure 2-2(b). Which reactor would require the smaller volume to achieve a conversion of 80%: a CSTR or a PFR? The entering molar flow rate and the feed conditions are the same in both cases.
Figure 2-2(b)

Step by Step Answer:






