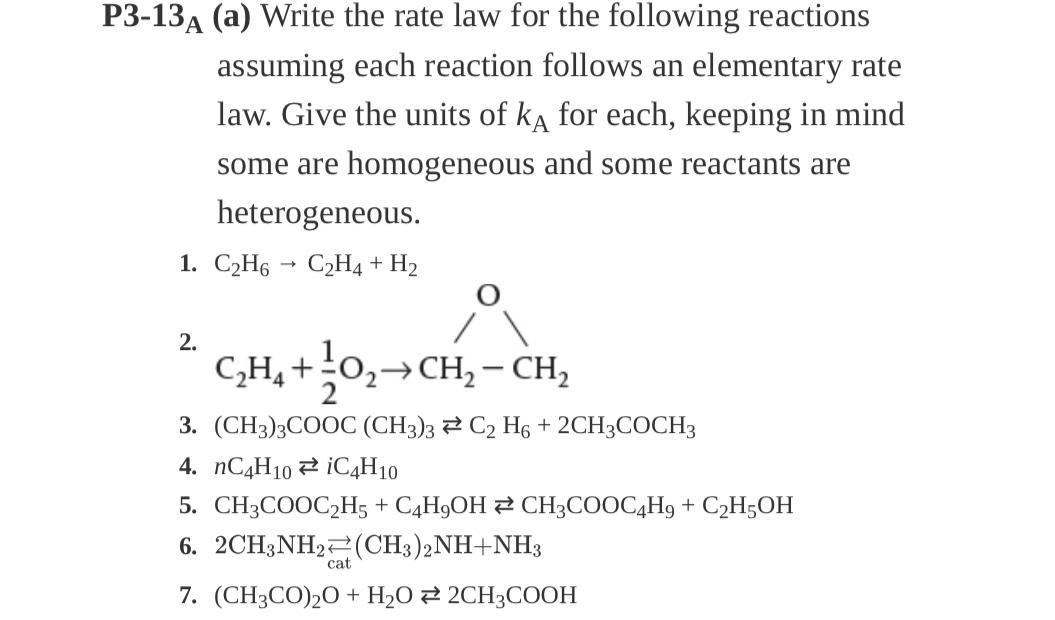
Question: P 3 - 1 3 A ( a ) Write the rate law for the following reactions assuming each reaction follows an elementary rate law.
A a Write the rate law for the following reactions assuming each reaction follows an elementary rate law. Give the units of for each, keeping in mind some are homogeneous and some reactants are heterogeneous.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


