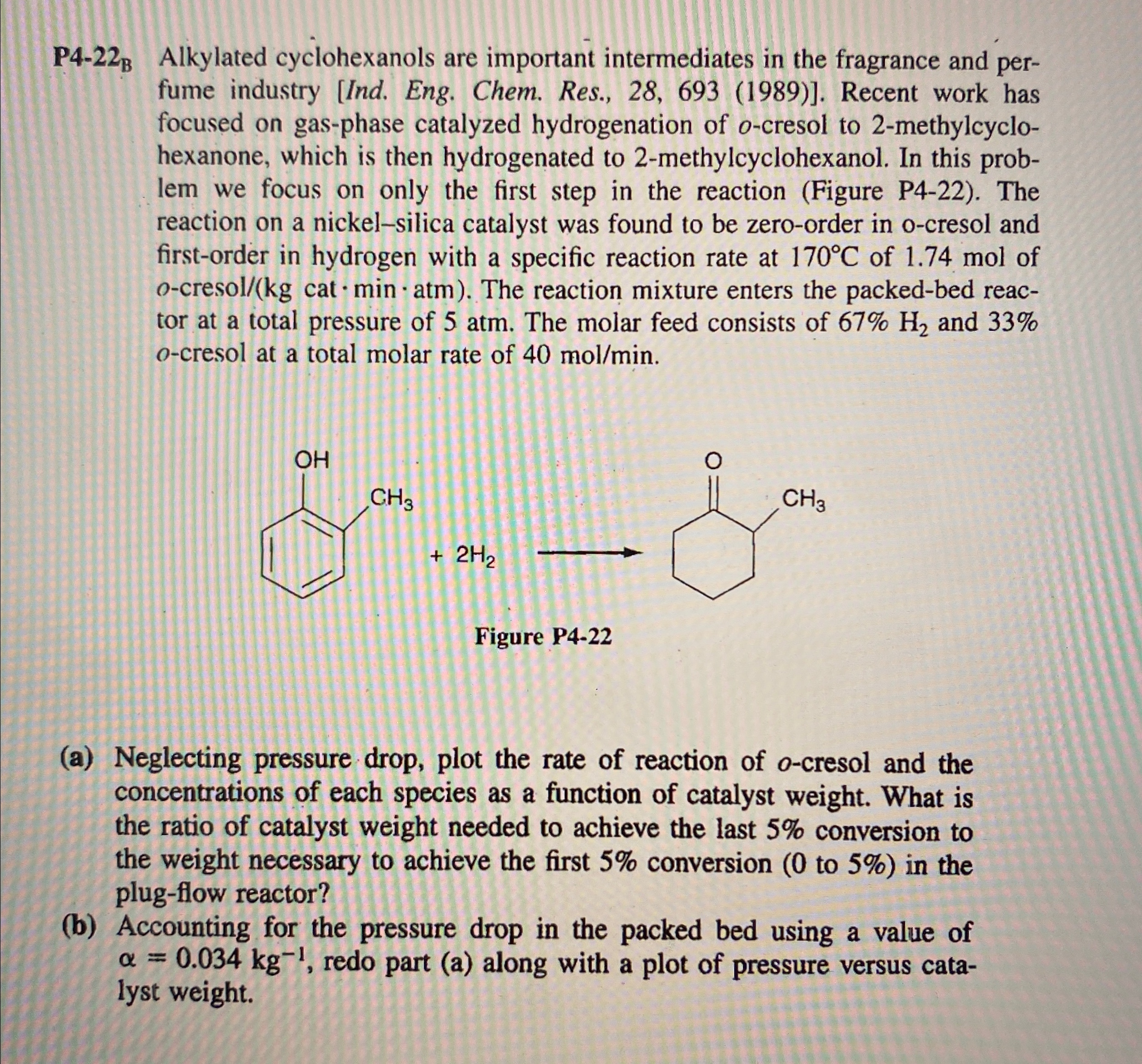
Question: P 4 - 2 2 Alkylated cyclohexanols are important intermediates in the fragrance and perfume industry [ Ind . Eng. Chem. Res., 2 8 ,
P Alkylated cyclohexanols are important intermediates in the fragrance and perfume industry Ind Eng. Chem. Res., Recent work has focused on gasphase catalyzed hydrogenation of cresol to methylcyclohexanone, which is then hydrogenated to methylcyclohexanol. In this problem we focus on only the first step in the reaction Figure P The reaction on a nickelsilica catalyst was found to be zeroorder in ocresol and firstorder in hydrogen with a specific reaction rate at of mol of cresol The reaction mixture enters the packedbed reactor at a total pressure of atm. The molar feed consists of and cresol at a total molar rate of
a Neglecting pressure drop, plot the rate of reaction of cresol and the concentrations of each species as a function of catalyst weight. What is the ratio of catalyst weight needed to achieve the last conversion to the weight necessary to achieve the first conversion to in the plugflow reactor?
b Accounting for the pressure drop in the packed bed using a value of redo part a along with a plot of pressure versus catalyst weight.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


