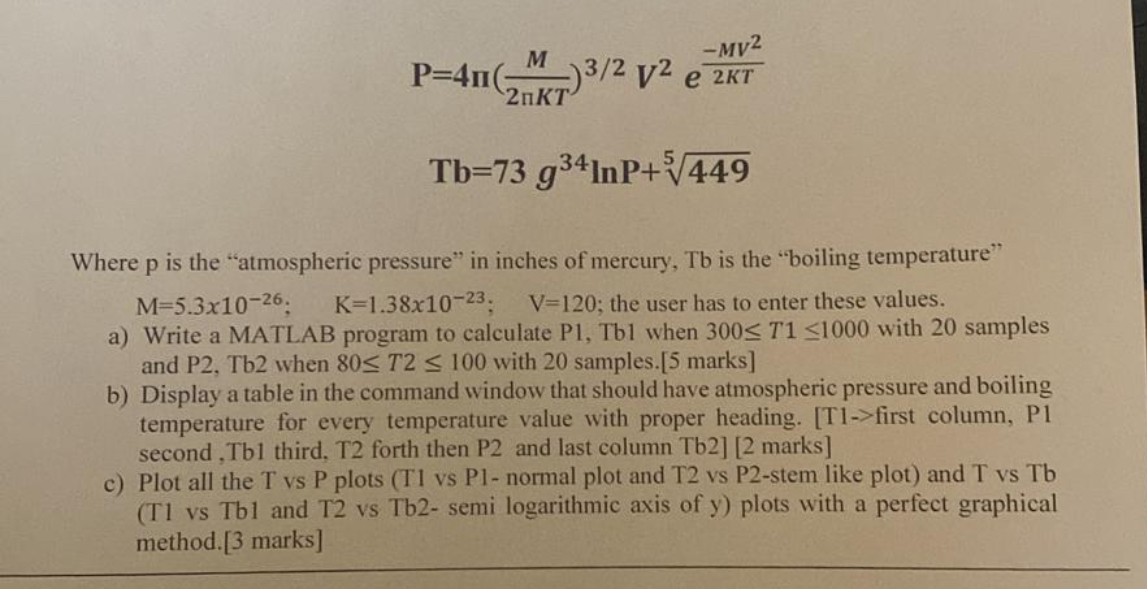
Question: P = 4 ( M 2 K T ) 3 2 V 2 e - M V 2 2 K T T b = 7
Where p is the "atmospheric pressure" in inches of mercury, Tb is the "boiling temperature" ;;; the user has to enter these values.
a Write a MATLAB program to calculate P Tb when with samples and P Tb when with samples. marks
b Display a table in the command window that should have atmospheric pressure and boiling temperature for every temperature value with proper heading. Tfirst column, P second, Tb third, T forth then P and last column Tb marks
c Plot all the T vs P plots T vs P normal plot and T vs Pstem like plot and T vs Tb T vs Tb and T vs Tbsemi logarithmic axis of y plots with a perfect graphical method. marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


