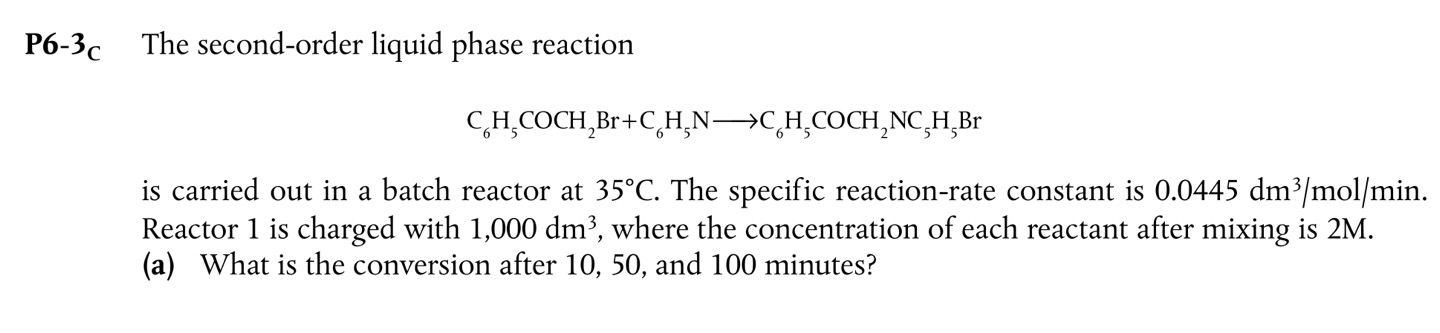
Question: P 6 - 3 C The second - order liquid phase reaction C 6 H 5 C O C H 2 B r + C
P C The secondorder liquid phase reaction
is carried out in a batch reactor at The specific reactionrate constant is Reactor is charged with where the concentration of each reactant after mixing is
a What is the conversion after and minutes?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


