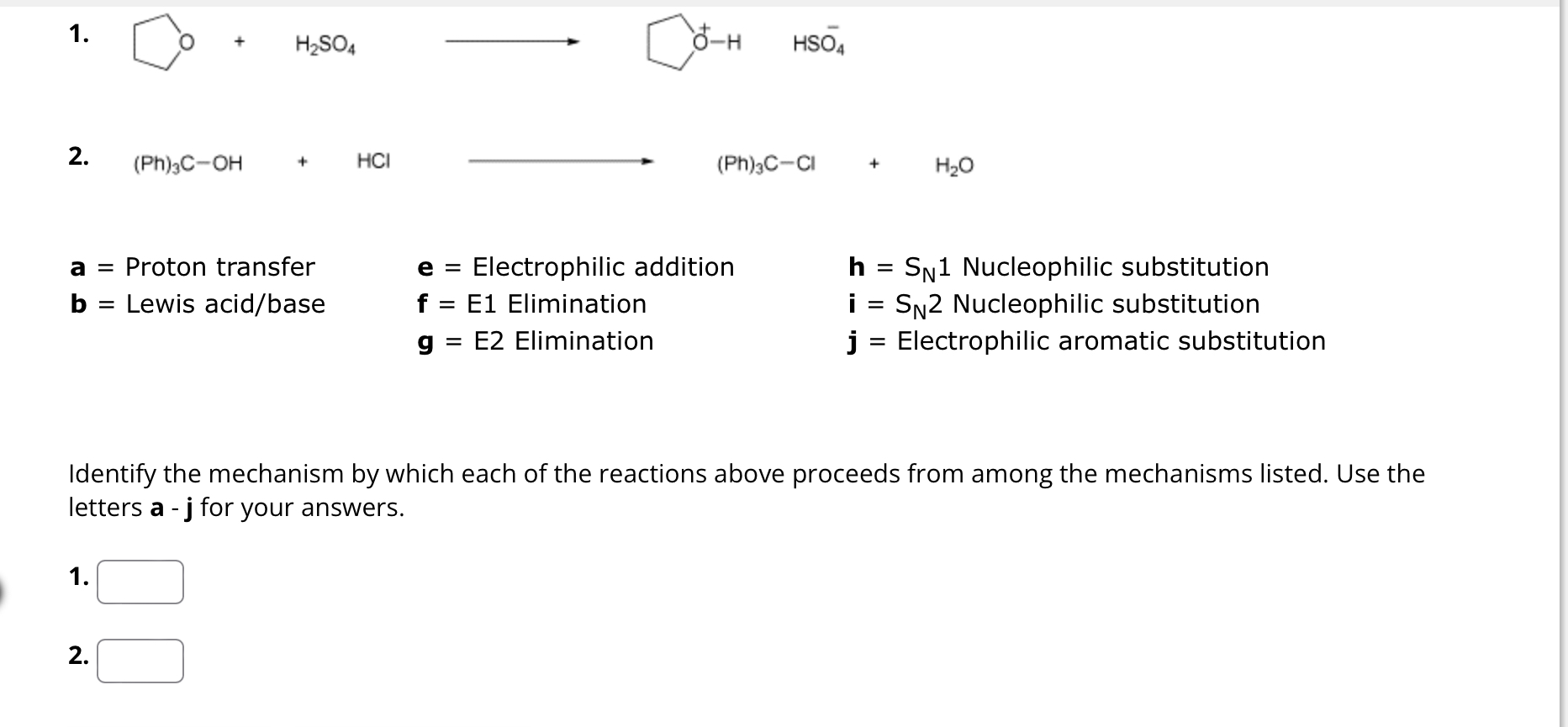
Question: ( P h ) 3 C - O H + HCllongrightarrow ( P h ) 3 C - C l + H 2 O a
HCllongrightarrow
Proton transfer
Lewis acidbase
Electrophilic addition
Elimination
E Elimination
Nucleophilic substitution
Nucleophilic substitution
Electrophilic aromatic substitution
Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the
letters for your answers.
Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the
etters for your answers.
longrightarrow
HCllongrightarrow
Proton transfer
Lewis acidbase
Electrophilic addition
Elimination
E Elimination
Nucleophilic substitution
Nucleophilic substitution
Electrophilic aromatic substitution
Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the
letters for your answers.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


