Question: p H = This question has multiple parts. Work all the parts to get the most points. What is the p H of a solution
This question has multiple parts. Work all the parts to get the most points.
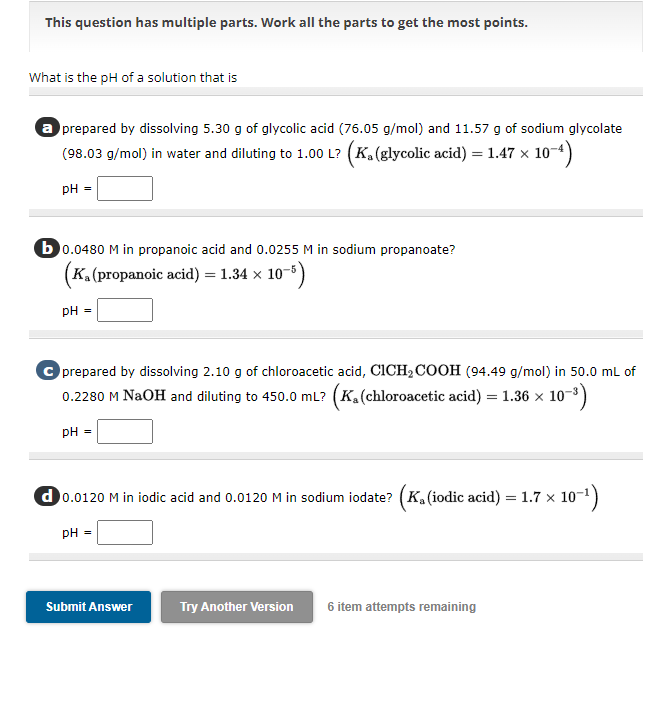
What is the of a solution that is
a prepared by dissolving of glycolic acid and of sodium glycolate
in water and diluting to glycolic acid :
b in propanoic acid and in sodium propanoate?
propanoic acid
C prepared by dissolving of chloroacetic acid, in of
MNaOH and diluting to chloroacetic acid :
d in iodic acid and in sodium iodate? iodic acid :
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


