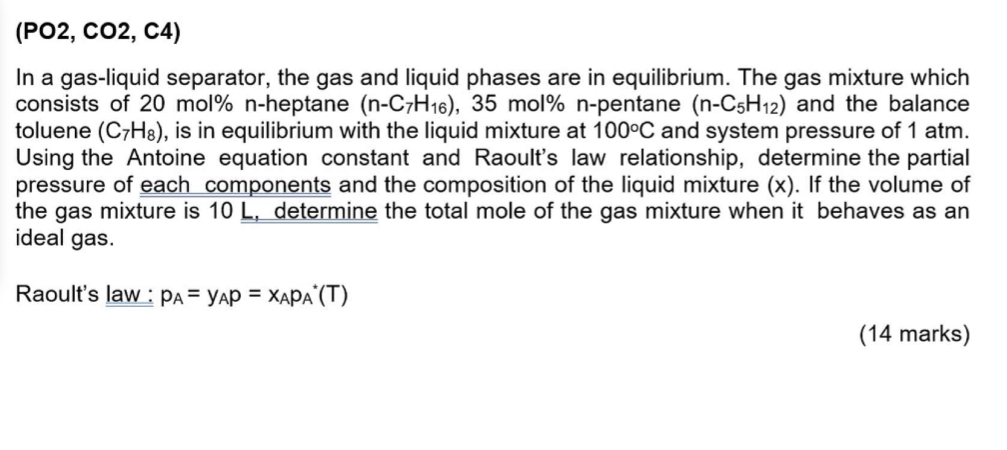
Question: ( P O 2 , C O 2 , C 4 ) In a gas - liquid separator, the gas and liquid phases are in
In a gasliquid separator, the gas and liquid phases are in equilibrium. The gas mixture which consists of mol nheptane molpentane and the balance toluene is in equilibrium with the liquid mixture at and system pressure of atm. Using the Antoine equation constant and Raoult's law relationship, determine the partial pressure of each components and the composition of the liquid mixture If the volume of the gas mixture is determine the total mole of the gas mixture when it behaves as an ideal gas.
Raoult's law:
marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


