Question: Question 4 (40 marks) Gas Compressor Feed Cooler Liquid Pump A two-phase mixture of benzene and hydrogen at 90C and 3 bar is cooled to
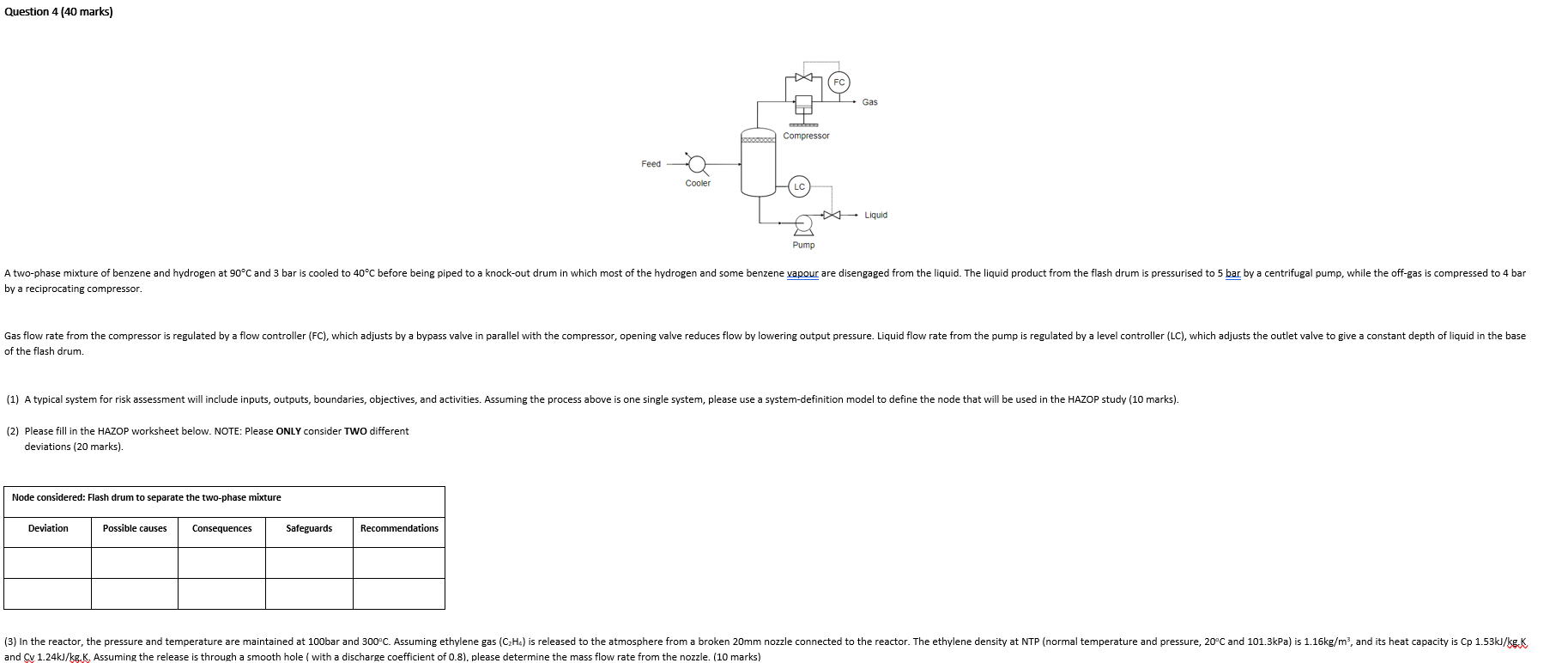
Question 4 (40 marks) Gas Compressor Feed Cooler Liquid Pump A two-phase mixture of benzene and hydrogen at 90C and 3 bar is cooled to 40C before being piped to a knock-out drum in which most of the hydrogen and some benzene vapour are disengaged from the liquid. The liquid product from the flash drum is pressurised to 5 bar by a centrifugal pump, while the off-gas is compressed to 4 bar by a reciprocating compressor. regulated by a level controller (LC), which adjusts the outlet valve to give a constant depth of liquid in the base Gas flow rate from the compressor is regulated by a flow controller (FC), which adjusts by a bypass valve in parallel with the compressor, opening valve reduces flow by lowering output pressure. Liquid flow rate from the pump of the flash drum. (1) A typical system for risk assessment will include inputs, outputs, boundaries, objectives, and activities. Assuming the process above is one single system, please use a system-definition model to define the node that will be used in the HAZOP study (10 marks). (2) Please fill in the HAZOP worksheet below. NOTE: Please ONLY consider TWO different deviations (20 marks). Node considered: Flash drum to separate the two-phase mixture Deviation Possible causes Consequences Safeguards Recommendations (3) In the reactor, the pressure and temperature are maintained at 100bar and 300C. Assuming ethylene gas (CzHc) is released to the atmosphere from a broken 20mm nozzle connected to the reactor. The ethylene density at NTP (normal temperature and pressure, 20C and 101.3kPa) is 1.16kg/m, and its heat capacity is Cp 1.53kJ/kgK and Cv 1.24kJ/kg.K. Assuming the release is through a smooth hole ( with a discharge coefficient of 0.8), please determine the mass flow rate from the nozzle. (10 marks) Question 4 (40 marks) Gas Compressor Feed Cooler Liquid Pump A two-phase mixture of benzene and hydrogen at 90C and 3 bar is cooled to 40C before being piped to a knock-out drum in which most of the hydrogen and some benzene vapour are disengaged from the liquid. The liquid product from the flash drum is pressurised to 5 bar by a centrifugal pump, while the off-gas is compressed to 4 bar by a reciprocating compressor. regulated by a level controller (LC), which adjusts the outlet valve to give a constant depth of liquid in the base Gas flow rate from the compressor is regulated by a flow controller (FC), which adjusts by a bypass valve in parallel with the compressor, opening valve reduces flow by lowering output pressure. Liquid flow rate from the pump of the flash drum. (1) A typical system for risk assessment will include inputs, outputs, boundaries, objectives, and activities. Assuming the process above is one single system, please use a system-definition model to define the node that will be used in the HAZOP study (10 marks). (2) Please fill in the HAZOP worksheet below. NOTE: Please ONLY consider TWO different deviations (20 marks). Node considered: Flash drum to separate the two-phase mixture Deviation Possible causes Consequences Safeguards Recommendations (3) In the reactor, the pressure and temperature are maintained at 100bar and 300C. Assuming ethylene gas (CzHc) is released to the atmosphere from a broken 20mm nozzle connected to the reactor. The ethylene density at NTP (normal temperature and pressure, 20C and 101.3kPa) is 1.16kg/m, and its heat capacity is Cp 1.53kJ/kgK and Cv 1.24kJ/kg.K. Assuming the release is through a smooth hole ( with a discharge coefficient of 0.8), please determine the mass flow rate from the nozzle. (10 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


