Question: P1. Circle the correct choice for each. Oxidation can be defined as when a compound both Forms more (C-H or C-O bonds) AND Reduces the
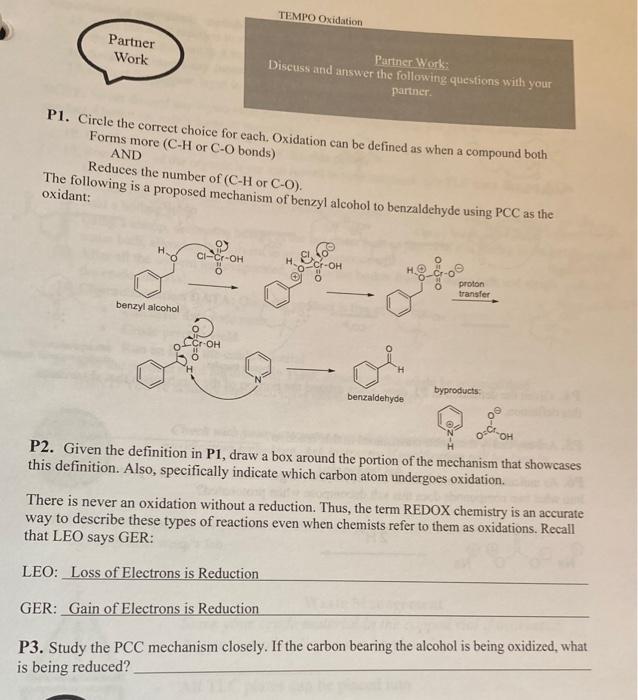
P1. Circle the correct choice for each. Oxidation can be defined as when a compound both Forms more (C-H or C-O bonds) AND Reduces the number of (C-H or CO). The following is a proposed mechanism of benzyl alcohol to benzaldehyde using PCC as the oxidant: benzyl aicohol benzaldehyde byproducts: P2. Given the definition in P1, draw a box around the portion of the mechanism that showcases this definition. Also, specifically indicate which carbon atom undergoes oxidation. There is never an oxidation without a reduction. Thus, the term REDOX chemistry is an accurate way to describe these types of reactions even when chemists refer to them as oxidations. Recall that LEO says GER: LEO: Loss of Electrons is Reduction GER: Gain of Electrons is Reduction P3. Study the PCC mechanism closely. If the carbon bearing the alcohol is being oxidized, what is being reduced
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


