Question: P5-9A MODIFIED) The liquid-phase reaction A + B + C follows an elementary rate law and is carried out isothermally in a batch system. How
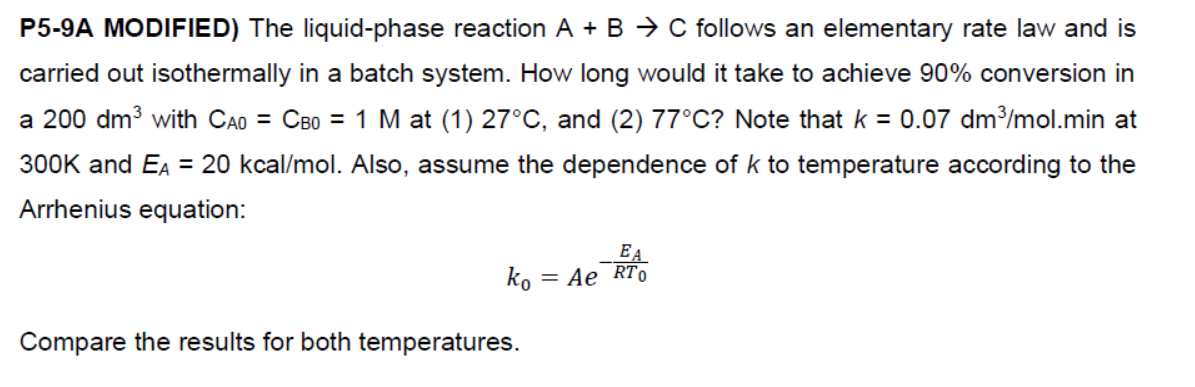
P5-9A MODIFIED) The liquid-phase reaction A + B + C follows an elementary rate law and is carried out isothermally in a batch system. How long would it take to achieve 90% conversion in a 200 dm3 with Cao = CBo = 1 M at (1) 27C, and (2) 77C? Note that k = 0.07 dm3/mol.min at 300K and EA = 20 kcal/ mol. Also, assume the dependence of k to temperature according to the Arrhenius equation: EA ko = Ae RTO Compare the results for both temperatures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


