Question: The liquid-phase reaction A +B - C follows an elementary rate law and is carried out isothermally in a flow system. The concentrations of
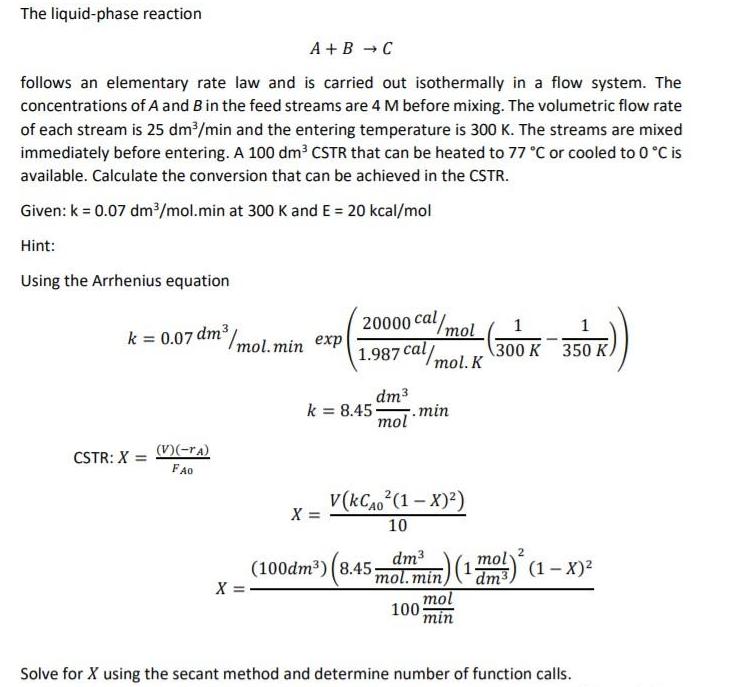
The liquid-phase reaction A +B - C follows an elementary rate law and is carried out isothermally in a flow system. The concentrations of A and Bin the feed streams are 4 M before mixing. The volumetric flow rate of each stream is 25 dm/min and the entering temperature is 300 K. The streams are mixed immediately before entering. A 100 dm2 CSTR that can be heated to 77 C or cooled to 0 C is available. Calculate the conversion that can be achieved in the CSTR. Given: k = 0.07 dm/mol.min at 300 K and E = 20 kcal/mol Hint: Using the Arrhenius equation 20000 cal/mol 1.987 cal/mol. K k = 0.07 dm /mol.min exp 300 K 350 K. dm3 k = 8.45. 7.min mol CSTR: X = V(-ra) FA0 v(KCA0 (1 X)) X = 10 dm3 (100dm) (8.45mol.min) mol dm3 mol (1m) (1 x)2 X = 100 min Solve for X using the secant method and determine number of function calls.
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
MATLAB CODE TO CALCULATE CONVERSION USING SECANT METHOD Code starts from here clc clear all Define F... View full answer

Get step-by-step solutions from verified subject matter experts


