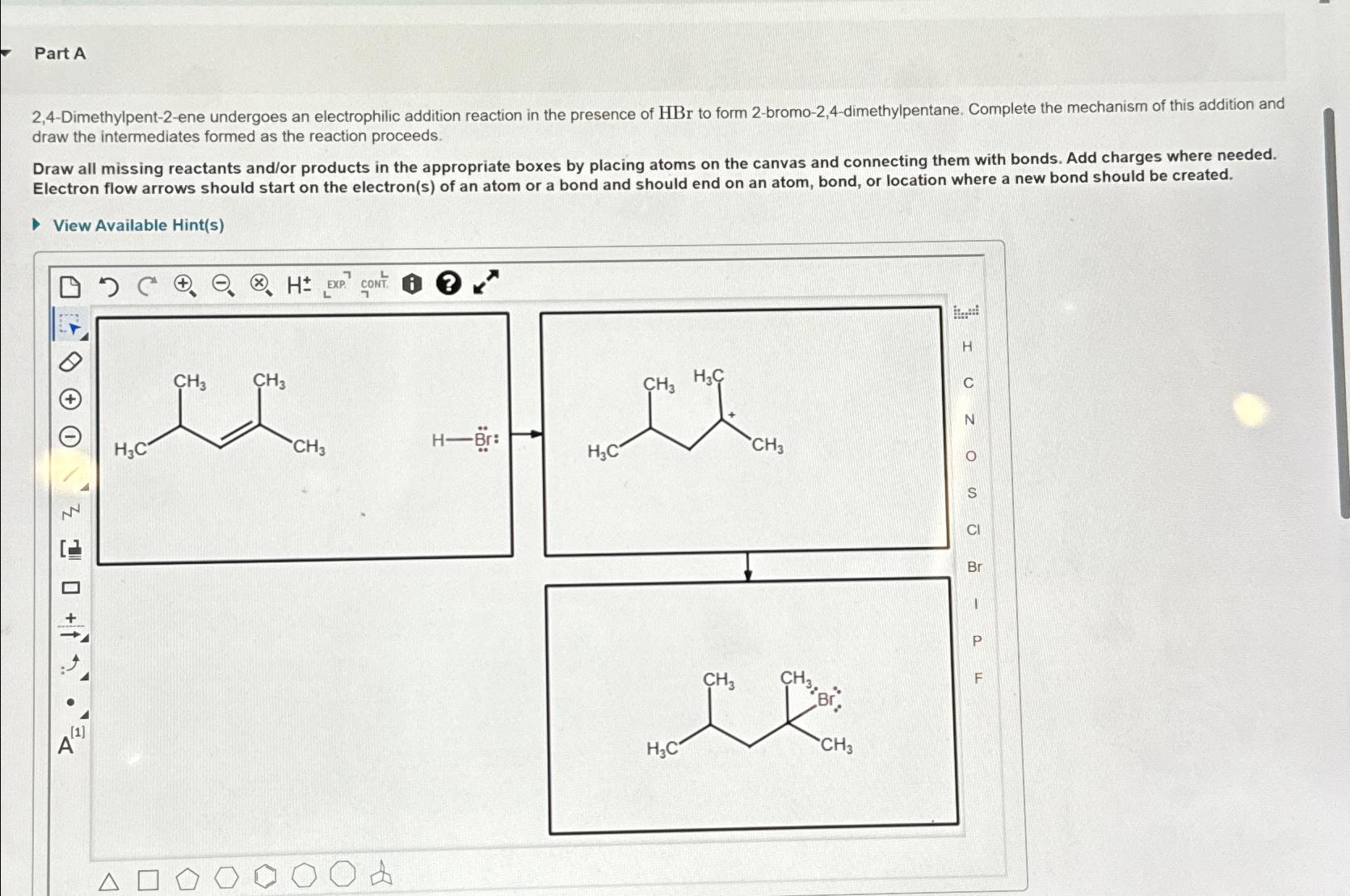
Question: Part A 2 , 4 - Dimethylpent - 2 - ene undergoes an electrophilic addition reaction in the presence of H B r to form
Part A
Dimethylpentene undergoes an electrophilic addition reaction in the presence of to form bromodimethylpentane. Complete the mechanism of this addition and draw the intermediates formed as the reaction proceeds.
Draw all missing reactants andor products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron flow arrows should start on the electrons of an atom or a bond and should end on an atom, bond, or location where a new bond should be created.
View Available Hints
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


