Question: Part A A solution is made containing 20.4 g phenol CH3OH) in 445 g ethanol (CH,CH,OH) Calculate the mole fraction of phenol Express the mole
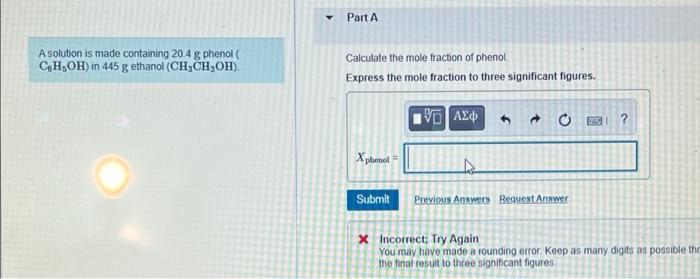

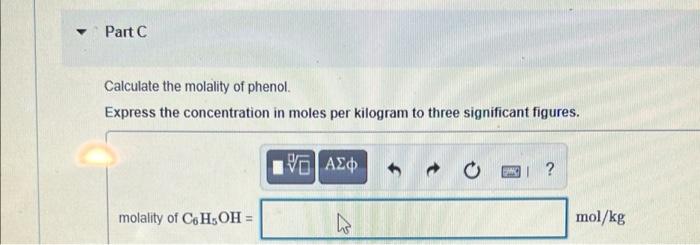
Part A A solution is made containing 20.4 g phenol CH3OH) in 445 g ethanol (CH,CH,OH) Calculate the mole fraction of phenol Express the mole fraction to three significant figures. 90 AED O ? Xpbenol = Submit Previous Ans Request Answer X Incorrect; Try Again You may have made a rounding error. Keep as many digits as possible the the final result to three significant figures Part B Calculate the mass percent of phenol Express the mass percent to three significant figures. V0 | ? mass % of C6H5OH = % Part C Calculate the molality of phenol. Express the concentration in moles per kilogram to three significant figures. IV AED ? molality of C6H5OH = ho mol/kg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


