Question: Part A Acetylene, C_(2)H_(2) , can be converted to ethane, C_(2)H_(6) , by a process known as hydrogenation. The reaction is C_(2)H_(2)(g)+2H_(2)(g)C_(2)H_(6)(g) Given the
Part A\ Acetylene,
C_(2)H_(2), can be converted to ethane,
C_(2)H_(6), by a process known as hydrogenation. The reaction is\
C_(2)H_(2)(g)+2H_(2)(g)C_(2)H_(6)(g)\ Given the following data at standard conditions (all pressures equal to
1atmand the common reference temperature
298K), what is the value of
K_(p)fo reaction?\ \\\\table[[Substance,\\\\table[[
\\\\Delta G_(f)\\\\deg 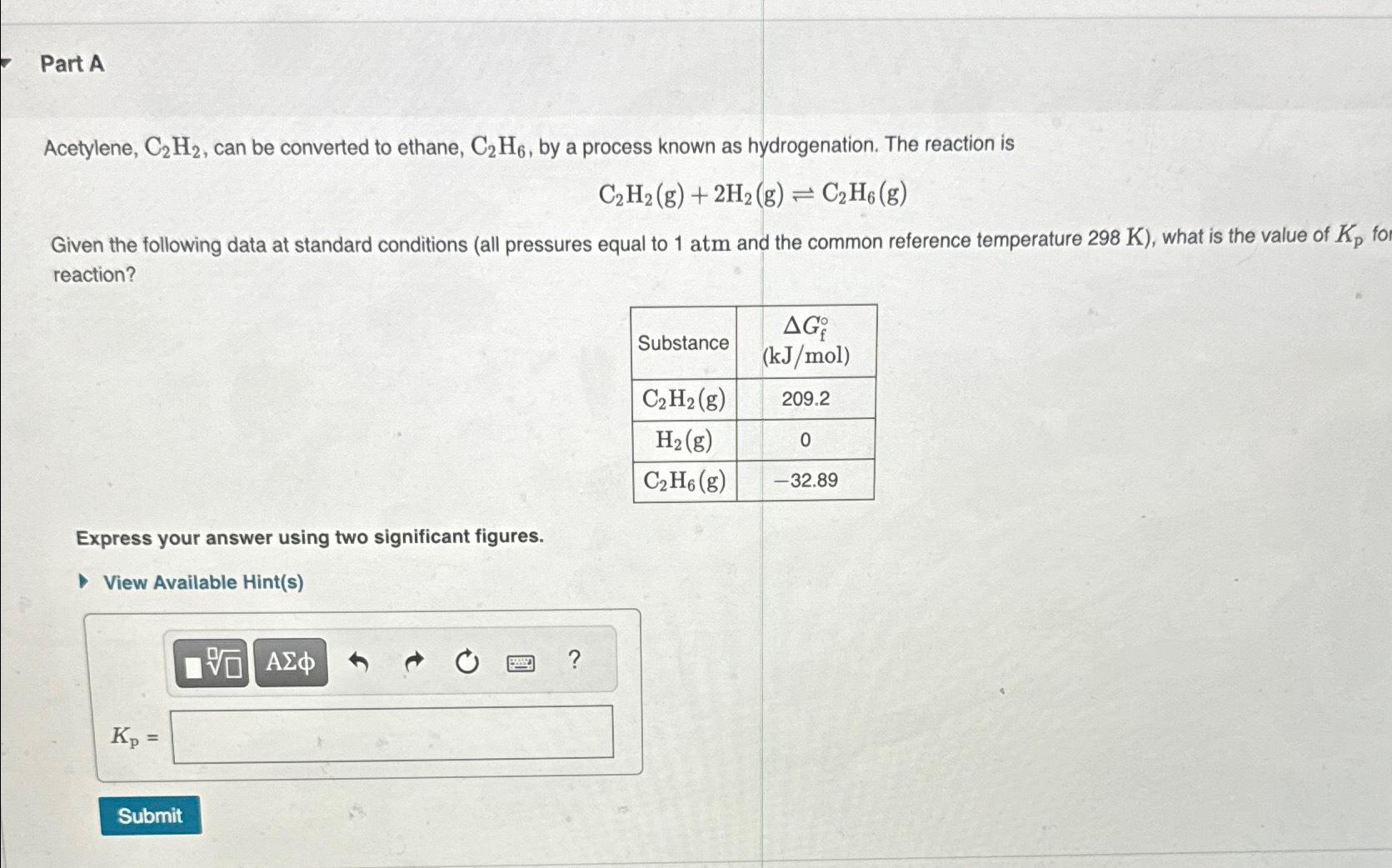
Acetylene, C2H2, can be converted to ethane, C2H6, by a process known as hydrogenation. The reaction is C2H2(g)+2H2(g)C2H6(g) Given the following data at standard conditions (all pressures equal to 1atm and the common reference temperature 298K ), what is the value of Kp for reaction? Express your answer using two significant figures. View Available Hint(s) Kp=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


