Question: Part a, b, c, and d please C. 50 g of liquid water at 0C is mixed with 50 g of ice at 0C. D.
Part a, b, c, and d please
C. 50 g of liquid water at 0C is mixed with 50 g of ice at 0C.
D. 50 g of liquid water at 20C is mixed with 50 g of ice at 20C.
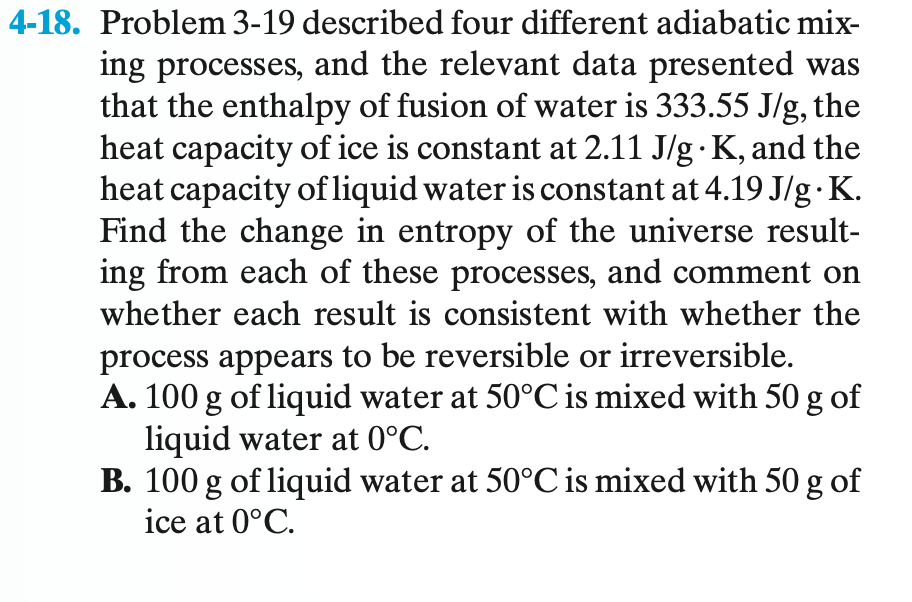
4-18. Problem 3-19 described four different adiabatic mix- ing processes, and the relevant data presented was that the enthalpy of fusion of water is 333.55 J/g, the heat capacity of ice is constant at 2.11 J/g.K, and the heat capacity of liquid water is constant at 4.19 J/g.K. Find the change in entropy of the universe result- ing from each of these processes, and comment on whether each result is consistent with whether the process appears to be reversible or irreversible. A. 100 g of liquid water at 50C is mixed with 50 g of liquid water at 0C. B. 100 g of liquid water at 50C is mixed with 50 g of ice at 0C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


